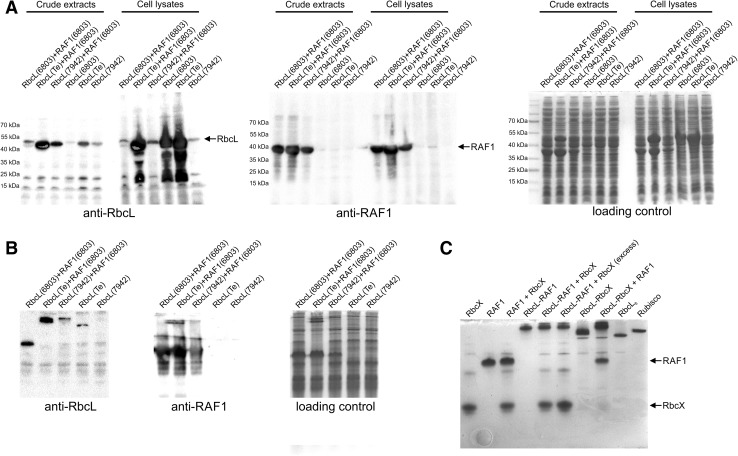
Fig. 6.
Determination of RAF1 chaperone activity toward cyanobacterial RbcL. a Co-expression of raf1 gene from Synechocystis sp. PCC 6803 with rbcL from Synechocystis sp. PCC 6803 (6803), Thermosynechococcus elongatus (Te) or Synechococcus elongatus PCC 7942 (7942) in E. coli cells. As a control, cells expressing rbcL genes alone were used. 20 µg of crude extracts as well as cell lysates from E. coli cells were separated under denaturing conditions (SDS-PAGE) and subjected to immunodetection using anti-RbcL or anti-RAF1 antibodies (a). Extracts with confirmed soluble RbcL presence were additionally separated under non-denaturing conditions (Native-PAGE) and probed with anti-RbcL or anti-RAF1 antibodies (b). c Identification of mutual RAF1-RbcL-RbcX interactions. A total of 2.5 µg (or 5 µg in lanes where “excess” is indicated) of RAF16803, RbcX6803 proteins as well as RbcLTe–RAF16803 or RbcLTe–RbcX6803 complex were loaded either directly or after pre-incubation with its putative interactor on non-denaturing gel and separated. As a control for complex migration, RbcL8 and Rubisco (RbcL8RbcS8) from T. elongatus were used. Positions of unbound RbcX and RAF1 are indicated with arrows. Addition of RAF1 resulted in an upshift of the RbcLTe–RbcX6803 complex, whereas no substitution of RAF1 in RbcLTe–RAF16803 by RbcX or direct interaction between RAF1 and RbcX was observed