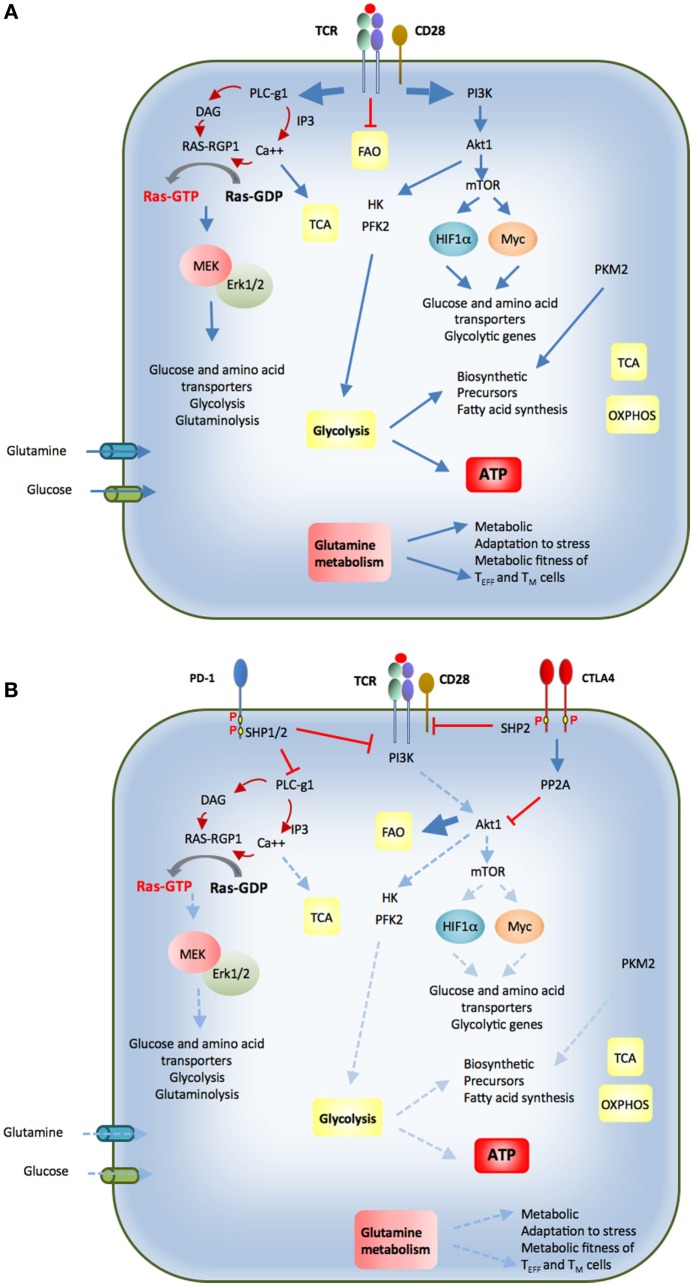
Figure 3.
(A) Upon antigen encounter T cells differentiate into effector cells. Antigen binding to the T cell receptor (TCR) and coactivation by CD28 inhibit fatty acid oxidation (FAO) and activate PI3K-Akt. This activation triggers glycolytic enzymes HK and PFK2. Additionally, mTOR signaling is turned on, which enhances expression of glycolytic genes, glucose, and amino acid transporters via activation of transcription factors HIF1α and Myc. Activation of PLC-γ1 and generation of second messengers result in activation of Ras and MEK/Erk pathway, which is required for expression of nutrient transporters and nutrient utilization. Calcium release activates calcium-dependent mitochondrial dehydrogenases, which activate the TCA cycle. Effector T cells also switch from balanced PKM1 and PKM2 expression to increased and predominant expression of PKM2, which promotes generation of biosynthetic precursors. These events promote glucose and glutamine uptake, increased glycolysis and glutaminolysis combined with a high degree of protein, lipid, and nucleic acid synthesis to support cell growth and proliferation. CD28 costimulation is required for activation of the signaling pathways that support these metabolic changes. (B) Cytotoxic T lymphocyte antigen-4 (CTLA-4) and programmed death-1 (PD-1) coinhibitory receptors are expressed in activated T cells. Via the recruitment of phosphatases, both these coinhibitory receptors attenuate the signaling events mediated by ligation of the TCR by antigen and have a mandatory role in the metabolic changes required for optimal T cell activation, function, and differentiation. CTLA-4 opposes the effects of CD28 costimulation and can inhibit potent TCR-mediated signals. PD-1 inhibits weak but not strong TCR signals. The imbalanced activation of these signaling pathways alters the metabolic reprogramming of T cells and their differentiation fate (see text for details).