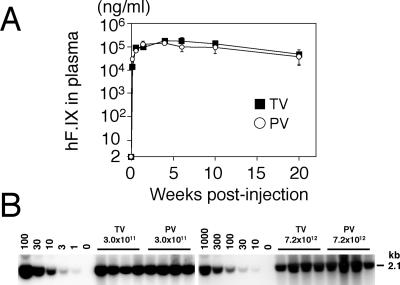
FIG. 4.
Comparison of efficiency of rAAV8-mediated liver transduction between tail vein and portal vein injections. (A) Plasma human coagulation factor IX (hF.IX) levels after tail vein (TV) or portal vein (PV) injection of AAV8-hF.IX16 into male C57BL/6 mice. Robust human coagulation factor IX expression with no lag phase was observed with both routes. Expression peaked 4 weeks after injection, followed by a substantial (≈75%) decline. Vertical bars indicate standard deviations. (B) Vector genome copy numbers (ds-vg/dge) in livers transduced with AAV8-EF1α-nlslacZ via tail vein or portal vein injection at 3.0 × 1011 or 7.2 × 1012 vg/mouse. Total liver DNA was extracted 6 weeks postinjection, and 10 μg of DNA was analyzed by Southern blot with BglI digestion and a 2.1-kb lacZ probe (BglI-BglI fragment). The left and right blots were analyzed separately with a different series of vector copy number standards. The double-stranded vector copy number standards (0 to 100 and 0 to 1,000 ds-vg/dge) were prepared by adding the corresponding amount of plasmid, pAAV-EF1α-nlslacZ, to 10 μg of liver DNA extracted from a naïve mouse. Each lane represents an individual mouse. Routes of administration and vector doses are indicated above the lanes.