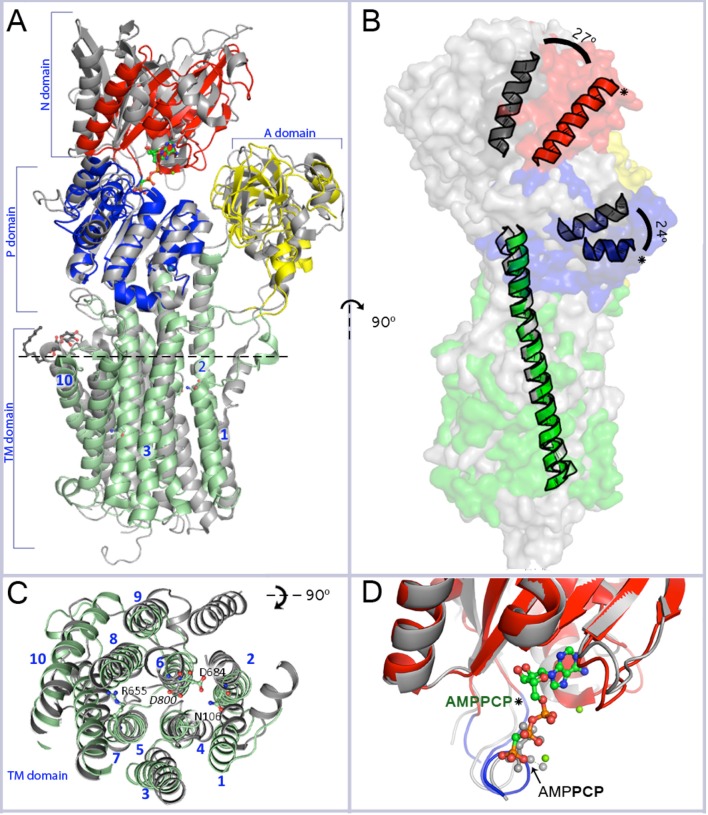
Figure 1.
Overall resemblance of AHA2-AMPPCP to the SERCA-SLN complex. (A) Superposition of the revised structure of AHA2-AMPPCP (domains colored as indicated) and the SERCA-SLN complex (gray; pdb id: 4H1W) both representing E1 state. The structures are superimposed on Cα position of the TM or P domains only. Mg2+ and K+ ions are shown by green and purple-blue spheres, respectively. The spatial organization of the cytoplasmic domains is similar in both E1 state structures. Two loops connecting the A-domain with TM1 and TM3, respectively, align well in AHA2 and SERCA, although for the latter TM1 is longer and kinks into a short helix parallel to the membrane. The TM3 connector forms a longer helix in the A-domain of SERCA compared to AHA2. The N-domain is moved slightly closer toward the A-domain in the SERCA structure. The adenine base part of the AMPPCP molecule is coordinated by side chains of Asp372 and Asp375 and the backbone oxygen of Ser457. The major differences between the P-domains are visible in a region between Pro533AHA2 to Asp559AHA2, which covers one loop and two short helixes. (B) Side view of the aligned pumps (superimposed on TM2–TM10 domains, r.m.s.d 3.07 Å for 159 Cα atoms) showing a tilt of the cytoplasmic headpiece of AHA2 (in colors) relatively to SERCA (gray). The N- and P-domain headpiece of AHA2 is tilted by ~25° (measured between Cα of Ser436AHA2 and Thr538SERCA for the N-domain and between Cα of Pro550AHA2 and Pro662SERCA for the P-domain, using Cα of Lys625AHA2 in TM5 in both cases as an apex). Asterisks mark helices from the P- and N-domain of AHA2. (C) Alignment of the transmembrane region. Top view from the plane marked by dashed line at segment A. Catalytically important residues of AHA2 and conserved Asp800 of SERCA are labeled. (D) Superposition of the N-domain of AHA2 and SERCA-SLN (r.m.s.d 0.97 Å for 123 atoms superimposed Cα atoms), both in E1 states and showing overlapping binding of β-γ phosphates of AMPPCP. The AMPCPP molecule represents the revised AHA2 model.