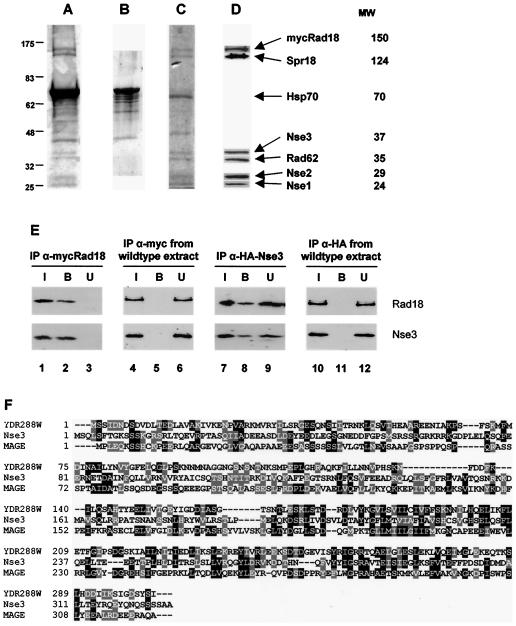
FIG. 4.
Purification and identification of Rad18 (Smc6)/Spr18 (Smc5) complex components. A lysate prepared from a Myc-tagged Rad18 (Smc6) strain was incubated overnight at 4°C with an anti-Myc antibody that was previously cross-linked to protein G-Sepharose beads. The beads were washed extensively, and bound proteins were eluted by incubation with an excess peptide corresponding to the Myc epitope, separated by SDS-PAGE, and stained with colloidal Coomassie blue (A). (B) The intense contaminating band at 70 kDa was identified as Hsp70 by mass spectrometry and was effectively removed by washing the beads with 5 mM ATP. (C) The remaining proteins were eluted as before and separated by SDS-PAGE, and individual bands were excised and identified by trypsin digestion and mass fingerprinting. (D) Antibodies were raised against Nse1, Nse2, Nse3, and Rad62 and were used, along with Rad18 (Smc6) and Spr18 (Smc5) antibodies, to confirm the presence of the identified proteins in an independent preparation of the complex by immunoblotting. (E) Lysates prepared from Myc-tagged Rad18 (Smc6) (lanes 1 to 3), HA-tagged Nse3 (lanes 7 to 9), or untagged (lanes 4 to 6 and 10 to 12) strains were incubated overnight at 4°C with either anti-Myc or anti-HA antibodies that were previously cross-linked to protein G-Sepharose beads. The beads were washed extensively, and the input (I), bound (B), and unbound (U) proteins were analyzed by SDS-PAGE and immunoblotting with anti-Rad18 and anti-Nse3 antibodies. (F) Alignment of Nse3, S. cerevisiae YDR288W, and the MAGE consensus sequence KOG4562.