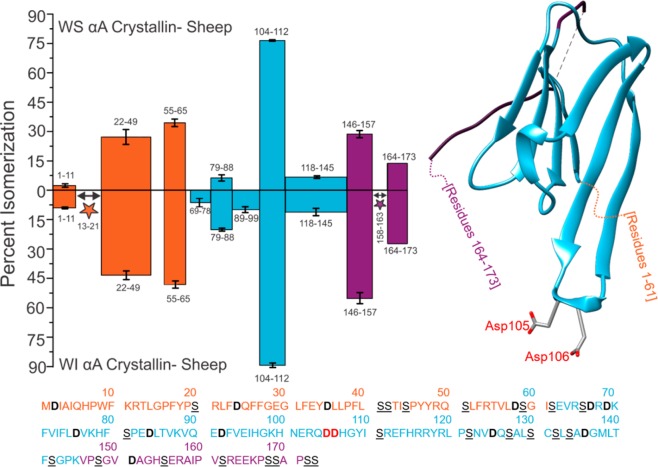
Figure 2.
Percent isomerization of water-soluble (WS) αA Sheep versus water-insoluble (WI) αA Sheep. Orange, disordered N-terminus; blue, structured α-crystallin domain; purple, disordered C-terminus. Three separate digests were performed; error bars represent standard deviations. Number ranges represent peptide sequences. Peptide 164–173 does not contain error bars because it only appeared baseline-resolved in one digest. The full protein sequence is given below the plot, with aspartic acid residues in bolded/black and serine residues in underlined/black. Asp105 and Asp106 are in bold red text in the amino acid sequence and are shown explicitly in the crystal structure (PDB 3L1F) to highlight an important region of isomerization. Stars indicate isomerized regions where isomerization was identified, but quantitation was not possible due to incomplete chromatographic separation.