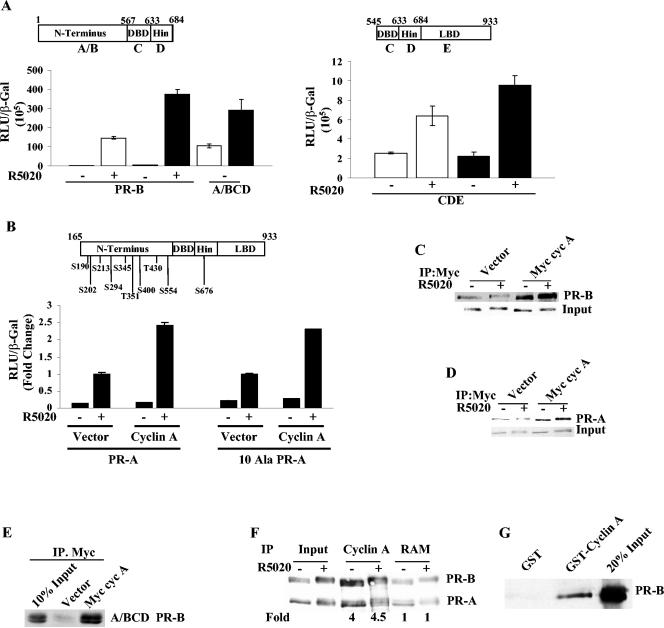
FIG. 2.
Functional and physical interactions between cyclin A and PR. (A) HeLa cells were cotransfected with 10 ng of full-length PR-B or PR-B constructs coding for the N-terminal DNA binding domain (DBD) hinge (left panel) or DBD hinge LBD (right panel), 0.5 μg of vector or cyclin A, 0.25 μg of GRE2-E1b-LUC, and 0.05 μg of β-Gal. The cells were treated or not with 10 nM R5020, and luciferase activity was normalized to β-Gal levels. White bars are vector-transfected samples, and black bars are cyclin A-transfected samples. Also shown are the schematic representations of the PR-B constructs used. (B) HeLa cells were transfected with 0.25 μg of GRE2-E1b-LUC, 10 ng of wild-type PR-A, or a 10 Ala mutant of PR-A (all of the serines and threonines in Ser/Thr-Pro motifs were mutated to alanines), and 0.5 μg of cyclin A or vector. The cells were treated with 10 nM R5020, and luciferase activity was measured and normalized to β-Gal levels. The top panel shows the sites that were mutated to alanine. The hormone-treated vector-transfected samples were set to 1 to facilitate a comparison of fold activation. The actual luciferase and β-Gal values for the hormone-treated wild-type and the 10 Ala mutant-transfected samples were 12.09 and 7.24, respectively. (C and D) HeLa cells were cotransfected with 0.25 μg of PR-B (C) or PR-A (D) and with 2.5 μg of myc-tagged cyclin A or pCMV vector. Twenty-four hours after transfection, the cells were treated with 10 nM R5020 for 60 min and harvested, and 100 μg of extract was incubated with myc 9E10 antibody as indicated in Materials and Methods. The immunoprecipitated protein extracts were run on an SDS-PAGE, and PR was detected with the 1294 antibody. (E) Cyclin A interacts with the N terminus of PR-B. HeLa cells were cotransfected with 0.25 μg of A/BCD PR-B and with 2.5 μg of myc-tagged cyclin A or backbone pCMV; immunoprecipitation and blotting were performed as described for panel C. (F) T47D cells were treated with or without 10 nM R5020 for 60 min, and the cell extracts were immunoprecipitated with cyclin A antibody or a nonspecific rabbit anti-mouse IgG (RAM) as described in Materials and Methods. The immunoprecipitated protein extract was run on an SDS-PAGE gel, and PR was detected using the PR antibody. The films were quantified densitometrically, and the fold change in PR-B immunoprecipitated by cyclin A antibody compared to the RAM-immunoprecipitated samples is given under each lane. The inputs in panels C to E were 10% of the total protein extract used for immunoprecipitation. (G) Cyclin A interacts with PR-B in vitro. One microgram of baculovirus-expressed GST-tagged cyclin A or GST was bound to glutathione-Sepharose beads, and then in vitro-translated [35S]PR-B was added. The beads were washed and eluted, the eluate was run with 20% input on an SDS-PAGE, and radiolabeled PR-B was detected by autoradiography. Myc cyc A, myc-tagged cyclin A; IP, immunoprecipitation; S, serine; T, threonine; DBD, DNA binding domain; Wt, wild type.