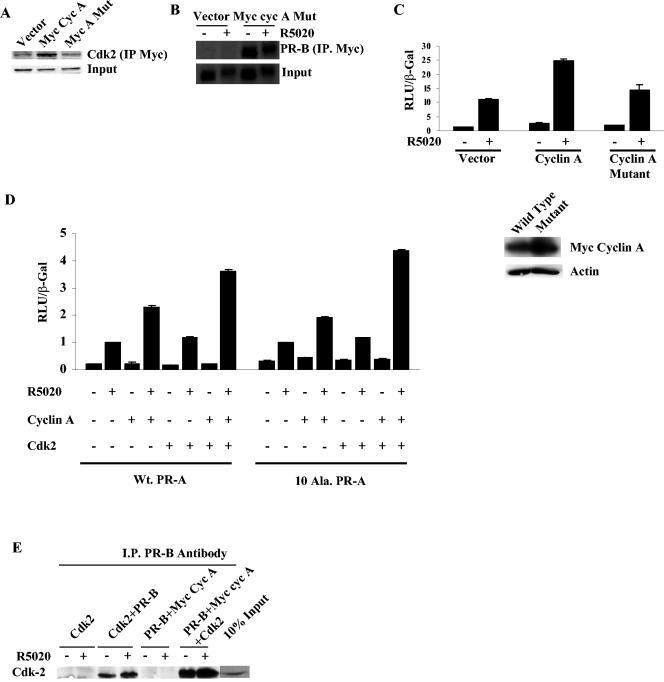
FIG. 4.
Cyclin A requires Cdk2 to stimulate PR activity but not for interaction with PR. (A) Myc-tagged cyclin A wild type or the Cdk2 binding mutant (2.5 μg) was cotransfected with Cdk2 (2.5 μg) into HeLa cells, and the cell extracts were immunoprecipitated with myc 9E10 antibody and blotted for Cdk2. (B) The Cdk2 binding mutant of myc-tagged cyclin A was cotransfected (2.5 μg) with 250 ng of PR-B, immunoprecipitated with myc 9E10 antibody, and blotted for PR. (C) Wild-type, Cdk2 binding mutant of cyclin A or vector backbone was cotransfected (0.5 μg) with 0.25 μg of GRE2-E1b-LUC and 10 ng of PR-B, luciferase activity was measured, and the values were normalized to β-Gal levels. The Western assay in the lower panel shows the expression of the myc cyclin A and the Cdk2 binding mutant of myc cyclin A. (D) HeLa cells were transfected as described in Materials and Methods with 0.25 μg of GRE2-E1b-LUC, 0.5 μg of cyclin A, Cdk2, or combinations of both, and 10 ng of PR-A or the phosphorylation-deficient mutant (10 Ala PR-A). The cells were treated with 10 nM R5020 for 24 h and harvested, and luciferase activity was measured and normalized to the β-Gal levels. The values of hormone-treated vector-transfected samples were set to 1. (E) Cdk2 interacts with PR-B, and the interaction is increased by transfection of cyclin A. HeLa cells were transfected with 0.25 μg of PR-B, 2.5 μg of Cdk2 or myc cyclin A, or a combination of both. After 24 h, the cells were treated with R5020 for 60 min. Protein extracts were immunoprecipitated with PR antibody and then blotted with Cdk2 antibody. I.P., immunoprecipitation; myc cyc A, myc-tagged cyclin A.