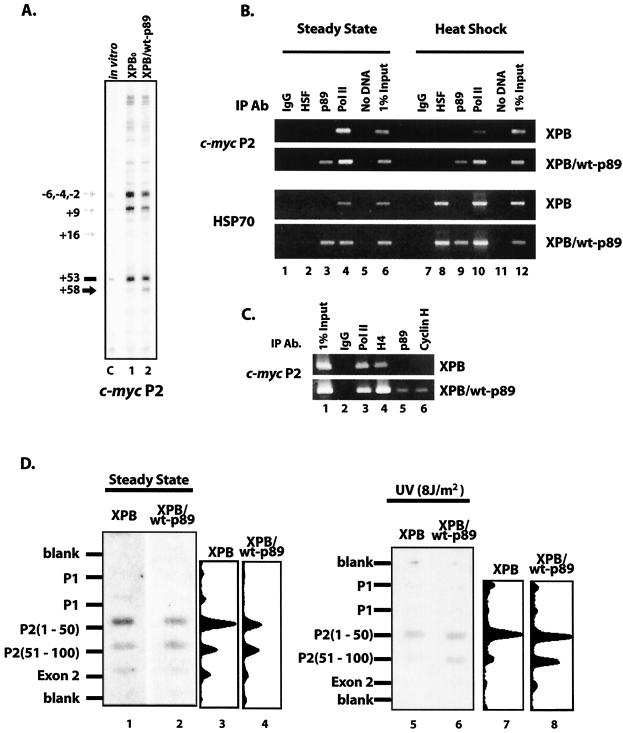
FIG. 5.
Holdback of c-myc transcription occurs in a broader zone in XPB/wt-p89 than in XPB cells. (A) Conformation-sensitive in vivo footprinting of the c-myc P2 promoter with KMnO4 shows that the region around +58 is hyperreactive (black arrow) in cells with wild-type p89; modest start-site hyporeactivity (grey arrows) is seen in XPB/wt-p89 versus XPB cells, consistent with a downstream shift of transcription complexes in XPB/wt-p89 cells. Blank regions are hyporeactive, due to a paucity of thymidines. Lane C, genomic DNA treated with KMnO4 in vitro for 1 min at 25°C. (B) ChIP analysis reveals diminished TFIIH binding at the c-myc P2 promoter in XPB cells, unless complemented with wild-type p89 (top panels, lanes 3); the same occurs at the hsp70 promoter (bottom panels, lanes 3). Binding of heat shock factor following heat shock is equivalent in both cells (lanes 8). Anti-p89 used in ChIP experiments recognizes both wild-type and truncated forms of p89. (C) ChIP analysis of the c-myc promoter in XPB and XPB/wt-p89 cells with α-p89 and α-CycH (Austral). (D) Nuclear run-on shows differential holdback at the major c-myc P2 promoter under steady-state conditions (left), as well as after UV irradiation (right). Hybridization with the second consecutive (downstream) P2 oligonucleotide is weaker for XPB cells, indicative of premature release of polymerase in cells with impaired TFIIH (lanes 1 and 3 versus 2 and 4). Differential release is exaggerated following UV irradiation (lanes 5 and 7 versus 6 and 8). Scans demonstrate relative intensities of the steady-state panel.