Abstract
Background.
Limited data are available on human papillomavirus (HPV) infection among human immunodeficiency virus (HIV)–negative or HIV-positive couples followed longitudinally.
Methods.
Genital HPV was assessed in 725 concordant HIV-negative couples and 209 HIV-positive couples enrolled in a male circumcision trial in Rakai, Uganda, using the Roche Linear Array assay, which detects 37 HPV genotypes. Human papillomavirus prevalence and determinants of genotype-specific concordance were assessed at annual visits. Cumulative detection of HPV genotypes over 2 years was also assessed.
Results.
At enrollment, HPV infection was detected in 54% of HIV-negative women, 56% of HIV-negative men, and 93% of HIV-positive men and women. For HIV-negative couples, genotypic concordance was 30% at baseline (n = 219/725) and declined significantly with age (adjusted prevelance risk ratio [adjPRR] = 0.53; 95% confidence interval [CI] = 0.28–0.93 comparing women aged >40 years to those aged 15–19 years) and male circumcision (adjPRR = 0.60; 95% CI = 0.47–0.77) and increased among couples with recent intercourse (adjPRR = 1.26; 95% CI = 1.04–1.53). These associations were not seen in HIV-positive couples. Among couples with HPV results at all visits, ≥1 of the same genotypes were detected in both partners in 60% of HIV-negative couples and 96% of HIV-positive couples over 2 years.
Conclusion.
Human papillomavirus genotype-specific concordance is more common in HIV-positive couples, and irrespective of HIV status, the majority of couples exhibit HPV concordance over 2 years.
Keywords: Human papillomavirus (HPV), concordance, male circumcision, HIV, Uganda, sexually transmitted infections.
Human papillomavirus (HPV) infection is common among sexually active individuals, and persistent high-risk HPV (HR-HPV) infection can lead to oral and anogenital cancers, including cervical cancer [1, 2]. Cervical and penile cancers are highest in sub–Saharan Africa where human immunodeficiency virus (HIV) and HR-HPV coinfections are common and cancer screening programs are largely unavailable [1–3]. Additionally, individuals with HIV have significantly higher rates of multiple HR-HPV infections and more rapid progression to neoplasia [3–5].
Numerous studies throughout North America, Europe, and Asia have assessed HPV concordance in HIV-negative couples [6–12]. Human papillomavirus concordance is typically defined as the simultaneous detection of the same HPV genotype in both sexual partners at a single time point. A meta-analysis of 30 studies of HPV concordance among HIV-negative heterosexual couples reported that 25.5% of couples are infected with ≥1 of same genotype [6].
Most data on HPV infection in couples come from cross-sectional studies, which likely underestimate HPV transmission events between partners [6]. This is because infection may be cleared or controlled in 1 or both partners before sampling or because low-level replicating virus is not detected by available assays. Moreover, the majority of HPV concordance studies have included couples within a limited age range or only individuals with HPV-associated diseases, and few studies have correlated HPV concordance with sociodemographic characteristics or sexual risk behaviors, which may affect transmission and persistent HPV detection in couples [6]. There is also a lack of data on HPV infection among couples, especially in Africa, where HIV and HPV disease burden is greatest. Only 2 cross-sectional studies in Africa have assessed HPV concordance in HIV-positive couples, and they have shown that HPV concordance is high with HIV coinfection [13, 14].
Analyses that assess HPV concordance in couples over time and link these events to HIV status and sociodemographic and sexual behavioral characteristics may provide insights into the natural history of HPV infection. Here, we assess trends and determinants of HPV concordance using longitudinal data collected over a 2-year period from concordant HIV-negative and -positive heterosexual couples aged 15–49 years.
MATERIALS AND METHODS
Study Population
Two parallel but independent trials of male circumcision for HIV/sexually transmitted infection prevention were conducted in Rakai, Uganda [15–18]. One trial only enrolled HIV-negative men, and the other trial primarily enrolled HIV-positive men. Human immunodeficiency virus–positive men with CD4 counts <350 cells/mm3 or World Health Organization stage 4 disease were excluded from the trials and referred for treatment. No follow-up CD4 testing was performed. Men were randomly assigned to receive immediate circumcision (intervention) or circumcision delayed for 24 months (control). Men who were married or in long-term consensual relationships were asked to identify their female partners; the latter were separately contacted and invited to participate in a follow-up study. Women were eligible for enrollment if their linked male partner was a trial participant and they were capable of providing informed consent. Written informed consent was provided by all participants at enrollment. Men and their female partners were enrolled between August 2003 and December 2006 and followed annually for 2 years (through 2008).
Couples were assessed for HPV as part of the trial. HPV was evaluated among 1097 heterosexual couples, consisting of 1011 men and 1097 women aged 15–49 years, as previously described [19]. The number of women exceeded the number of men because of polygamy. This study was restricted to the couples (n = 934) who had HPV results available for both partners at any of the study visits: baseline (n = 759), 12-month visit (n = 492), or 24-month visit (n = 536). We also evaluated HPV concordance over the full 2-year period in couples who completed all 3 visits (n = 263). We excluded visits if either partner seroconverted to HIV or had indeterminate HIV test results because of the known association between HIV and HPV acquisition [20], and these incident HIV events were too infrequent for stratified analyses. Thus, couples in the analysis were either concordant HIV-negative (n = 725) or concordant HIV-positive (n = 209).
Infectious disease testing (HPV and HIV), physical examinations, and interviews to ascertain sociodemographic characteristics and sexual risk behaviors were conducted at baseline and at 12- and 24-months follow-up. All subjects were offered free HIV counseling and testing, health education, and condoms at each visit. All participants found to be HIV-positive were referred for free care to the Rakai Health Sciences Program HIV care and treatment services funded by the President’s Emergency Plan for AIDS Relief.
The trials were approved by the HIV Subcommittee of the Ugandan National Council for Science and Technology (Kampala, Uganda), and by 3 institutional review boards: the Science and Ethics Committee of the Uganda Virus Research Institute (Entebbe, Uganda), the Johns Hopkins University Bloomberg School of Public Health Institutional Review Board (Baltimore, MD), and the Western Institutional Review Board (Olympia, WA). The trials were overseen by independent data safety monitoring boards [15–18], and were registered with Clinical.Trials.Gov numbers NCT00425984 and NCT00124878.
Laboratory Methods
Penile swab samples for HPV detection were obtained by trained clinicians using a standardized procedure. Moistened Dacron swabs were rotated around the circumference of the penis at the coronal sulcus and placed in a Digene specimen transport medium. Female partners were asked to provide self-administered vaginal swabs for HPV detection. Women were instructed to squat, insert a 20-cm Dacron or cotton-tipped swab and rotate the swab high in the vaginal vault. After collection, women handed the swab to a field worker who placed the swab in specimen transport medium. Specimen collection was well accepted, with compliance rates >90%, and self-collected vaginal swabs were comparable to physician-collected cervical swabs for HPV detection [21].
Human papillomavirus genotyping was performed using the Roche HPV Linear Array, which detects 37 HR-HPV and low-risk HPV (LR-HPV) genotypes. Human papillomavirus genotypes 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 were considered HR-HPV genotypes. Swabs with no detectable cellular beta-globin or detectable HPV were excluded because the adequacy of the sample collection could not be ensured for these samples (0.1% of female swabs; 18.5% of male swabs).
Swab and serum samples were stored at −80°C until evaluated. Human immunodefiency virus status was determined using 2 separate enzyme-linked immunosorbent assays and confirmed by HIV-1 Western blot [15].
Statistical Analysis
We assessed the prevalence of concordant HPV infection with any HPV genotype in all 934 couples at their first study visit. Concordant HPV infection was defined as both the man and the woman having ≥1 of the same HPV genotypes detected. Concordant HPV infection with high- and low-risk genotype was also separately assessed. All results were reported in 5-year age groups using the female partner’s age.
We assessed whether HIV, sociodemographic factors, and behavioral factors were associated with genotype-specific HPV concordance at a given study visit, including data from all 3 visits (baseline, 12 months, and 24 months), among couples with HPV detected. The unit of observation was an HPV genotype detected in at least 1 partner at a couple visit. Couples with multiple HPV genotypes at a given visit could contribute multiple observations for each genotype detected at that visit, and couples with the same genotype-specific infection at all 3 visits contributed 3 observations to the analysis. The primary outcome was genotype-specific HPV concordance defined as detection of the HPV genotype in both partners at the same visit (conversely, a nonevent was discordant HPV infection in either the man or the woman). Analyses were stratified by HIV status. The relationship between genotype-specific HPV concordance and female age in years was assessed using a generalized additive binomial model (mgcv package in R version 3.2). Associations between genotype-specific HPV concordance and categorical and binary variables were reported as prevalence risk ratios (PRRs) and 95% confidence intervals (CIs), which were estimated using modified Poisson regression with generalized estimating equations and robust variance estimators. Risk factors for HPV concordance identified in prior studies (eg, age, male circumcision status [16]) or those associated with concordant HPV detection with a P value < .10 in the univariate analysis were entered into Poisson multivariable models.
Lastly, we examined cumulative detection of HPV genotypes over the 2-year study among couples who had HPV assessments available at all 3 study visits (n = 263). In this analysis, we defined concordant HPV detection as both partners having ≥1 of the same HPV genotype detected at any visit up to that time point (ie, cumulative HPV concordance). For example, if a woman had detectable HPV-16 at her baseline visit and her male partner had detectable HPV-16 at his 12 month visit, but not his baseline visit, that couple would be considered concordant HPV positive for HPV-16 at 12 months but not at baseline regardless of the woman’s HPV status at 12 months. We report cumulative HPV concordance to any HPV genotype from baseline through 24 months. We examined whether sociodemographic or sexual risk behaviors were associated with genotype-specific cumulative HPV concordance over 24 months using univariate and multivariate Poisson models with generalized estimating equations and robust variance estimators. We also assessed the associations between the total number of HPV genotypes detected in either partner relative to the total number of genotypes detected in both partners through 24 months using linear regression models stratified by HIV and male circumcision status.
All analyses were performed using the R statistical software (version 3.2).
RESULTS
Of 1097 couples assessed for HPV, 934 couples (725 HIV-negative and 209 HIV-positive) had at least 1 study visit where both partners were assessed for HPV infection at the same study visit. These 934 couples consisted of 870 men and 934 women due to polygamy. There were 263 (218 HIV-negative and 45 HIV-positive) couples who had HPV assessments at all 3 study visits. Table 1 shows characteristics at the enrollment visit when HPV was assessed in both partners, stratified by HIV status of the couple. The median age of HIV-negative men was 29 years (interquartile range [IQR] = 25–35), and the median age of their female partners was 25 years (IQR = 22–30) (Table 1). The HIV-positive couples tended to be older, with a median age 34 years (IQR = 30–38) for HIV-positive men and a median age of 28 years (IQR = 25–34) for their female partners. The median sex difference in age was 3 (IQR = 1–6) years in HIV-negative couples and 4 (IQR = 2–9) years in HIV-positive couples. Approximately half of male partners were in the intervention arm of the circumcision trial. Although the proportion of men reporting nonmarital sexual partners in the past year was similar between HIV-negative and HIV-positive men, the number of lifetime sexual partners was higher among HIV-positive men (P < .001).
Table 1.
Selected Baseline Characteristics of Heterosexual Couples With a Human Papillomavirus Assessment at >1 Study Visits (n = 934) and Couples With Human Papillomavirus Assessments at All 3 Study Visits (n = 263) Stratified by Couple Human Immunodeficiency Virus Serostatus
| All couples (n = 934) | Couples with all follow-up visits (n = 263) | |||
|---|---|---|---|---|
| HIV−/HIV− (n = 725) | HIV+/HIV+ (n = 209) | HIV−/HIV− (n = 218) | HIV+/HIV+ (m = 45) | |
| Male partner characteristics | No. (%) / median (interquartile range) | |||
| Intervention arm | 381 (53%) | 116 (56%) | 97 (44%) | 26 (58%) |
| Age, y | 29 (25–35) | 34 (30–38) | 30 (25–35) | 32 (27–36) |
| Polygamousa | 130 (18%) | 45 (22%) | 47 (22%) | 7 (16%) |
| Nonmarital sexual partnership in the last year | 235 (32%) | 73 (35%) | 71 (33%) | 19 (42%) |
| Number of sex partners in the last year | 1 (1–2) | 2 (1–2) | 2 (1–2) | 1 (1–2) |
| Number of lifetime sex partners | 5 (3–10) | 10 (6–20) | 6 (4–10) | 8 (6–15) |
| Consistent condom use in the last yearb | 26 (1.8%) | 13 (6.3%) | 4 (1.4%) | 0 (0.0%) |
| Self-reported genital ulcer disease in the last year | 58 (8.0%) | 59 (28%) | 20 (9.0%) | 6 (13%) |
| Female partner characteristics | ||||
| Age, y | 25 (22–30) | 28 (25–34) | 26 (22–30) | 26 (23–31) |
| Nonmarital sexual partnership in the last year | 28 (3.9%) | 10 (4.9%) | 9 (4.1%) | 1 (2.2%) |
| Number of sex partners in the last year | 1 (1-1) | 1 (1-1) | 1 (1-1) | 1 (1-1) |
| Number of lifetime sex partners | 2 (1–3) | 3 (2–4) | 2 (1–3) | 3 (2–4) |
| Consistent condom use in the last yearb | 2 (0.0%) | 15 (7.2%) | 1 (0.0%) | 0 (0.0%) |
| Self-reported genital ulcer disease in the last year | 105 (15%) | 62 (30%) | 28 (13%) | 12 (27%) |
aNumber (percentage) of men with more than one wife or long-term consensual partner.
bRefers to consistent condom use in the last year with all sexual partners.
Baseline Prevalence of Human Papillomavirus Infection in 934 Heterosexual Rakai Couples
At baseline, 54% (n = 395/725) of HIV-negative women and 56% (n = 407/725) of their HIV-negative male partners had ≥1 detectable HPV infections compared with 93% (n = 194/209) of HIV-positive women and 93% (n = 194/209) of their male partners. Among couples with evidence of HPV infection, the median number of detected HPV genotypes at enrollment was 2 (IQR = 1–3) in both HIV-negative women and men, 4 (IQR = 2–6) in HIV-positive women, and 5 (IQR = 2–8) in HIV-positive men. Overall, 30% (n = 219/725) of HIV-negative couples and 76% (n = 158/209) of HIV-positive couples were concordant for ≥1 HPV genotypes at baseline.
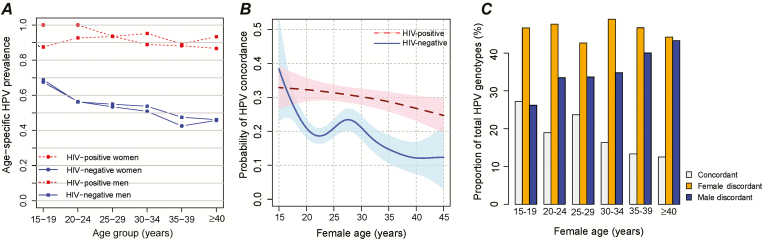
Prevalence of any detectable HPV infection (either HR-HPV or LR-HPV) at baseline was highest in women aged 15–19 years and in men aged 20–24 years (Figure 1A) and was higher in all age groups among HIV-positive compared with HIV-negative persons. Human papillomavirus prevalence declined with older age. Age-specific trends were similar when analyses were conducted separately for HR-HPV (Supplementary Figure 1A) and LR-HPV (Supplementary Figure 1B).
Figure 1.
Genotype-specific human papillomavirus (HPV) concordance in human immunodeficiency virus (HIV)–negative and HIV-positive couples in Rakai, Uganda. A, Age-specific HPV (including high-risk HPV [HR-HPV] and low-risk HPV [LR-HPV]) prevalence in women and their male partners. B, Probability that an HPV genotype detected in 1 partner was detected in the other partner by age of the female partner in years in HIV-negative couples (control arm only) and HIV-positive couples. C, Proportion of total HPV genotypes detected in HIV-negative couples (control arm only) that were detected in both partners (ie, concordant), the female partner only (ie, female discordant), and the male partner only (ie, male discordant).
Risk Factors for Genotype-Specific Human Papillomavirus Concordance at the Same Study Visit
There were 570 HIV-negative couples with at least 1 member of the couple having at least 1 detectable HPV genotype (n = 2752) during the 3 study visits. Univariate and multivariate associations between sociodemographic and sexual behaviors and genotype-specific HPV concordance at the same visit are shown in Table 2 for the HIV-negative couples. Overall, 20.5% (n = 564/2752) of HPV genotypes detected in 1 partner were also detected in the other partner at the same study visit. The probability that an HPV infection was detected in both partners at the same visit did not differ between HR-HPV and LR-HPV genotypes, male age, age difference between partners, lifetime number of sexual partners, nonmartial sexual partners, or self-reported genital ulcer disease in either sex.
Table 2.
Univariate and Multivariate Analyses of Possible Factors Associated With Genotype-Specific Human Papillomavirus Concordance at the Same Study Visit Among 570 Concordant Human Immunodeficiency Virus–Negative Couples With Detectable Human Papillomavirus Infection
| Viral and participants characteristics | HPV genotypes detected in both partners/HPV genotypes detected in either partner, No. (%) | PRR (95%CI) | P value | adjPRR (95% CI) | P value |
|---|---|---|---|---|---|
| Total | 564/2752 (20.5) | ||||
| High-risk genotype | |||||
| No | 325/1579 (20.6) | 1.00 (referent) | … | ||
| Yes | 239/1173 (20.4) | 0.99 (.86–1.14) | .89 | ||
| Female age, y | |||||
| 15–19 | 105/386 (27.2) | 1.00 (referent) | … | 1.00 (referent) | … |
| 20–24 | 198/1046 (18.9) | 0.69 (.52–.91) | .008 | 0.68 (.52–.89) | .005 |
| 25–29 | 171/722 (23.7) | 0.84 (.64–1.12) | .25 | 0.85 (.66–1.16) | .25 |
| 30–34 | 61/374 (16.3) | 0.64 (.44–.93) | .02 | 0.67 (.46–.96) | .03 |
| 35–39 | 16/120 (13.3) | 0.45 (.26–.78) | .004 | 0.49 (.29–.84) | .008 |
| ≥40 | 13/104 (12.5) | 0.51 (.27–.95) | .03 | 0.53 (.28–.93) | .045 |
| Male age, y | |||||
| 15–19 | 13/67 (19.4) | 0.90 (.47–1.71) | .75 | ||
| 20–24 | 139/584 (23.8) | 1.00 (referent) | … | ||
| 25–29 | 216/956 (22.6) | 0.96 (.73–1.24) | .07 | ||
| 30–34 | 99/576 (17.2) | 0.74 (.54–1.01) | .06 | ||
| 35–39 | 58/336 (17.3) | 0.78 (.54–1.12) | .18 | ||
| ≥40 | 39/233 (16.7) | 0.74 (.49–1.13) | .17 | ||
| Age difference in years, man relative to woman | |||||
| ≥2 y younger | 51/221 (23.1) | 0.93 (.64–1.35) | .71 | ||
| Within 1 y | 129/563 (22.9) | 1.00 (referent) | … | ||
| 2–4 y older | 190/948 (20.0) | 0.85 (.65–1.10) | .22 | ||
| 5–9 y older | 145/760 (19.1) | 0.91 (.70–1.20) | .52 | ||
| ≥10 y older | 59/260 (18.9) | 0.87 (.61–1.25) | .47 | ||
| Sex in the last 7 d | |||||
| No | 167/975 (17.1) | 1.00 (referent) | … | 1.00 (referent) | … |
| Yes | 397/1777 (22.3) | 1.21 (1.00–1.46) | .046 | 1.26 (1.04–1.53) | .02 |
| Female lifetime sex partners | |||||
| 1 | 195/935 (20.9) | 1.00 (referent) | … | 1.00 (referent) | … |
| 2 | 179/838 (21.4) | 0.95 (.75–1.21) | .69 | 0.95 (.76–1.19) | .68 |
| 3 | 119/620 (19.2) | 0.92 (.71–1.20) | .54 | 0.93 (.20–1.20) | .58 |
| ≥4 | 68/340 (20.0) | 0.87 (.64–1.20) | .40 | 0.86 (.65–1.16) | .33 |
| Unknown (>3) | 3/17 (17.6) | 0.78 (.66–.93) | .006 | 0.82 (.58–.91) | .006 |
| Male lifetime sex partners | |||||
| 1–2 | 64/262 (19.6) | 1.00 (referent) | … | ||
| 3–5 | 229/959 (23.9) | 1.28 (.92–1.78) | .15 | ||
| 6–10 | 122/683 (17.9) | 1.00 (.70–1.44) | .98 | ||
| ≥10 | 132/630 (21.0) | 1.11 (.78–1.59) | .57 | ||
| Unknown (>3) | 17/154 (11.0) | 0.62 (.31–1.24) | .18 | ||
| Self-reported genital ulcer in last year | |||||
| Neither partner | 422/2076 (20.3) | 1.00 (referent) | … | ||
| Woman only | 96/387 (24.9) | 1.23 (.97–1.56) | .09 | ||
| Man only | 31/201 (15.4) | 0.73 (.47–1.16) | .18 | ||
| Both partners | 14/72 (19.4) | 0.99 (.57–1.72) | .99 | ||
| Sex with outside partnersa | |||||
| Neither partner | 245/1203 (20.4) | 1.00 (referent) | … | ||
| Woman only | 19/109 (17.4) | 0.88 (.54–1.42) | .59 | ||
| Man only | 271/1312 (20.7) | 1.03 (.85–1.24) | .78 | ||
| Both partners | 28/112 (25.0) | 1.20 (.76–1.94) | .43 | ||
| Male circumcisedb | |||||
| No | 480/2158 (22.2) | 1.00 (referent) | … | 1.00 (referent) | … |
| Yes | 84/594 (14.1) | 0.62 (.48–.79) | <.001 | 0.60 (.47–.77) | <.001 |
Abbreviations: adjPRR, adjusted prevalence risk ratio; CI, confidence interval; HPV, human papillomavirus; PRR, prevalence risk ratio.
aOutside partnerships included other marital partnerships (polygamous unions) and nonmarital partnerships. bMale circumcision (intervention arm) was analyzed as a time-varying covariate.
The likelihood of genotype-specific HPV concordance at a visit was significantly lower among couples with a circumcised male (adjPRR = 0.60; 95% CI = .47–.77) and in couples with older female partners (>40 years compared with 15–19 years: adjPRR = 0.53; 95% CI = .28–.93). Restricting analysis to the uncircumcised control arm of the trial, the decline in HPV concordance among HIV-negative couples was primarily driven by an increase in the overall proportion of male HPV discordant infections (ie, HPV infections detected in the male partner, but not the female partner) (Figure 1C). Human papillomavirus concordance was significantly more common among couples who reported sexual intercourse within the last 7 days (adjPRR = 1.26; 95% CI = 1.04–1.53).
Of the 209 HIV-positive couples assessed for HPV, 207 (99%) couples had detectable HPV infection in at least 1 partner at ≥1 study visits. Genotype-specific HPV concordance was significantly higher in these HIV-positive couples compared with HPV-infected HIV-negative couples: 30.2% (n = 652/2157) of HPV genotypes detected in 1 partner were also detected in the other partner at the same study visit. (PRR = 1.44; 95% CI = 1.25–1.66). Circumcision and older female age were not associated with reduced genotype-specific HPV concordance in HIV-positive couples (Supplementary Table 1 and Figure 1B).
Cumulative Probability of Human Papillomavirus Concordance in Couples Over 2 Years
Among HIV-negative couples with HPV assessments at all 3 study visits (n = 238), the proportion of couples who ever had detectable infection with ≥1 of the same HPV genotypes increased from 32% at enrollment to 60% by year 2 (Table 3). The number of genotypes detected in both partners also increased from a mean and standard deviation of 0.5 ± 0.8 genotypes at baseline to 1.2 ± 1.6 by year 2. Among HIV-positive couples, 76% had genotypic concordance on their first visit, and this increased to 91% and 96% by 12 and 24 months, respectively. The total number of genotypes detected also increased in HIV-positive couples from 2.1 ± 1.8 genotypes at the first visit to 4.1 ± 2.8 by year 2. Overall, the probability that any given genotype detected in 1 partner was also detected in the other partner over the 2 years of follow-up was 27% (n = 265/990) in HIV-negative couples and 43% (n = 186/430) in HIV-positive couples.
Table 3.
Cumulative Detection of Human Papillomavirus Among 218 Concordant Human Immunodeficiency Virus–Negative Couples and 45 Concordant Human Immunodeficiency Virus–Positive Couples With Complete Follow-up From Baseline Through 2 Years
| HPV detected | HIV−/HIV− (n = 218) | HIV+/HIV+ (n = 45) | ||||
|---|---|---|---|---|---|---|
| Baseline | Year 1 | Year 2 | Baseline | Year 1 | Year 2 | |
| No detectable HPV in either partner, no. (%) | 51 (23%) |
29 (13%) | 22 (11%) | 1 (2%) |
0 (0%) |
0 (0%) |
| Any HPV genotype detected in either the male or female partner, no. (%) | 167 (77%) | 189 (86%) | 196 (89%) | 44 (97%) |
45 (100%) | 45 (100%) |
| ≥1 of the same HPV genotype detected in both partners, no. (%) | 70 (32%) | 110 (50%) | 130 (60%) | 34 (76%) |
41 (91%) | 43 (96%) |
| Total number of HPV genotypes detected in the male and female partner, mean (SD) | 2.4 (2.5) | 3.5 (3.3) | 4.5 (3.9) | 6.4 (3.7) | 8.5 (4.1) | 9.6 (4.4) |
| Total number of HPV genotypes detected in both partners, mean (SD) | 0.5 (0.8) | 0.9 (1.3) | 1.2 (1.6) | 2.1 (1.8) | 3.4 (2.4) | 4.1 (2.8) |
Abbreviations: HIV, human immunodeficiency virus; HPV, human papillomavirus; SD, standard deviation.
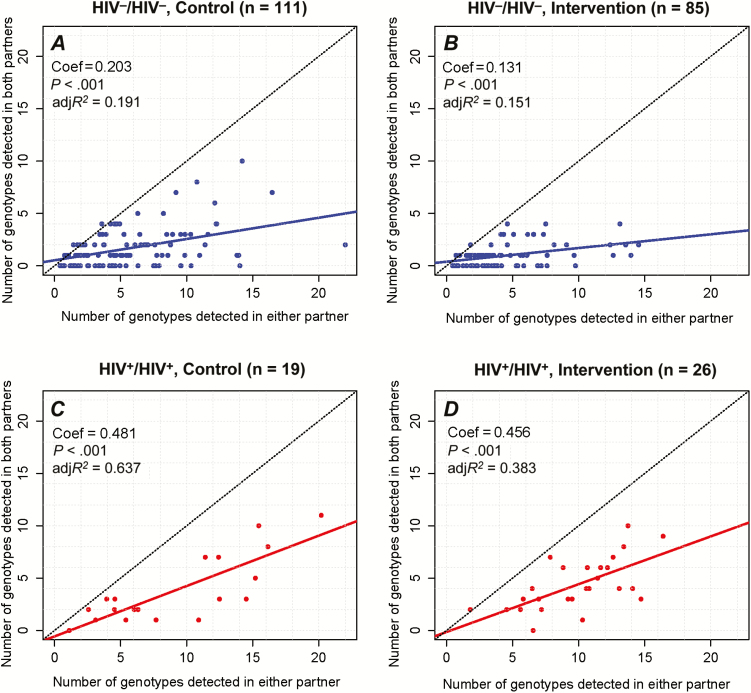
Figure 2 shows the total number of genotypes detected in both the male and female partners compared with the number of genotypes detected in both partners at any point over the 2-year period, stratified by HIV status and the male circumcision intervention and control arms. The total number of HPV genotypes detected in both partners significantly increased with the overall total number of genotypes detected in either the male or female partner, regardless of trial arm or HIV status. However, there were significantly greater numbers of concordant HPV infections in HIV-positive couples and in couples with an uncircumcised male partner regardless of the total number of HPV genotypes detected.
Figure 2.
Total number of human papillomavirus (HPV) genotypes detected in both the male and female partner versus the total number of HPV genotypes detected in both partners over a 2-year period (baseline, 12-month, 24-month visits) among 238 couples in the randomized trials of male circumcision. Analyses were conducted separately for human immunodeficiency virus (HIV)–negative couples in the control arm (A), HIV-negative couples in the circumcision intervention arm (B), HIV-positive couples in the control arm (C), and HIV-positive couples in the circumcision intervention arm of the trial (D).
DISCUSSION
This study shows that HPV concordance is associated with HIV infection and that cumulative incidence of genotype-specific HPV concordance over a 2-year period is high among male and female heterosexual partners regardless of HIV serostatus. In addition, visit-specific HPV concordance decreases with age in HIV-negative couples, which is predominantly driven by an increase in male HPV-positive discordance.
We and other have previously demonstrated that male circumcision decreases HPV prevalence in both HIV-negative and HIV-positive men [16, 22–27]. In addition, male circumcision decreases transmission of HPV to female partners among HIV-negative men, but not HIV-positive men [17, 28]. Male circumcision decreases the HPV load among both HIV-negative men and their female partners [29, 30]. Using data from the male circumcision trials, it has also been shown that HPV load is predictive of HPV transmission between heterosexual partners and the rate of new HPV detection among men and women is almost identical among HIV-negative couples [19]. However, we have never evaluated HPV concordance, the impact of longitudinal sampling on cumulative concordance, or the effect of recent sexual intercourse on HPV detection or directly compared the impact of HIV infection on couples’ HPV status.
All previous studies of HR-HPV concordance among heterosexual couples have focused on HIV-negative individuals, except for 2 studies from Africa [13, 14]. We show here that HIV infection was among the strongest risk factors for HPV concordance. Previous studies have shown increased prevalence of HPV infection in HIV-positive men and women, especially with multiple genotypes [5, 31]. This may be because HIV-positive individuals are more likely to acquire HPV due to more frequent exposure to HPV-infected partners and/or because HPV infections are less likely to be cleared or controlled among HIV-infected individuals with an impaired immunological response.
The probability that an HPV genotype detected in 1 partner was also detected in the other partner at the same study visit was 20.5% among HIV-negative couples, similar to the 25.5% concordance in a meta-analysis of studies from Europe, North America, and Asia [6]. We also found that HPV concordance is more common among younger couples, as reported previously [7]. Human papillomavirus concordance was higher among couples reporting sexual intercourse within the past week, and previous studies have shown that vaginal intercourse within 2 days prior to swab collection is associated with increased concordance due to possible contamination from the sexual partner [7, 32], which could explain the association with recent sex and HPV concordance in this study. Lastly, we observed increased HPV discordance among couples with a circumcised male partner, consistent with studies that showed that male circumcision decreases HR-HPV prevalence and incidence and increases clearance (ie, loss of HPV detection) in both men and their female partners [16, 17, 22–25].
Most studies that assess HPV concordance in couples have been cross-sectional [6]. In this study, we assessed HPV longitudinally over 2 years. The proportion of couples who had at least 1 HPV genotype detected in both partners nearly doubled from 32% at baseline to 60% cumulatively by year 2 in HIV-negative couples and was nearly universal among HIV-positive couples. These longitudinal data suggest that the prevalence of concordant HPV infection in couples is markedly underestimated with cross-sectional assessments. It is likely that more frequent HPV assessments over longer time periods would reveal even great levels of HPV concordance.
Human papillomavirus detection may represent newly acquired HPV infection or reactivation of controlled, or latent, HPV infection [33]. The high cumulative concordance observed in couples may thus result from frequent reinfection between partners or from periodic, nonsynchronous reactivation of infections over time. It is difficult using existing technologies and long interval-sampled study designs to differentiate between these non–mutually exclusive possibilities. However, the observed decline of HPV concordance with increasing age of the female partner among HIV-uninfected couples but not HIV-infected couples may provide insight into some of the sex variability in HPV epidemiology. The age-specific decline in couple concordance appears to be driven by higher HPV prevalence in the male partner, suggesting that women may develop better control of HPV infection than their male counterparts.
There are limitations with this study. Although this study includes sociodemographic and sexual risk behavior data, this information is always susceptible to recall bias. We used an HPV linear array assay, which is highly sensitive, detects 37 genotypes, and can quantify the viral load. However, because of concerns about adequacy of sample collection from men with keratinized epithelium, we were conservative in our estimates of detection, which were restricted to samples with amplifiable cellular or viral DNA. We only evaluated HPV at the coronal sulcus of the penis and the cervical fornix. However, there is high level of concordance between anogenital sites of the same individual [34, 35]. We also included male circumcision in the multivariate analyses because we have previously demonstrated that beta-globin detection is significantly lower among circumcised men versus uncircumcised men [16], which could affect our classification of concordant relationships. The findings are not applicable to all couples with HIV because only men with CD4 cell counts ≥350 cells/mL and no evidence of AIDS-related illnesses were enrolled into the trials. Our results were robust to adjustment for behavioral and clinical variables that may have confounded the associations with concordance among heterosexual partners.
In conclusion, HIV infection is positively associated with genotype-specific HPV concordance among heterosexual couples in Africa, and the probability of genotype-specific HPV concordance decreases significantly with female age in HIV-negative couples but not in HIV-positive couples. Our findings highlight the need for additional studies on HPV transmission, control, and reactivation. In addition, the high prevalence of HPV concordance among heterosexual couples suggests that all future interventions to lower the HPV burden of disease in Africa, such as vaccination, should focus on men as well as women prior to sexual debut.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We are most grateful to the study participants and the Rakai Community Advisory Board whose commitment and cooperation made this study possible.
Financial support. The trials were funded by the National Institutes of Health (NIH; U1AI51171) and Bill and Melinda Gates Foundation (22006.02).The National Institute of Allergy and Infectious Diseases (NIAID), NIH grants 1K23AI093152-01A1, U01-AI-068613, and 3U01-AI075115-03S1, and the Division of Intramural Research, NIAID, provided laboratory support. A. A. R. T. was supported by the NIH (1K23AI093152-01A1). M. K. G was supported by the NIH (T32AI102). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. de Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 2007; 7:453–9. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–917. [DOI] [PubMed] [Google Scholar]
- 3. Clifford GM, Gonçalves MA, Franceschi S, HPV and HIV Study Group Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS 2006; 20:2337–44. [DOI] [PubMed] [Google Scholar]
- 4. Harris TG, Burk RD, Palefsky JM, et al. Incidence of cervical squamous intraepithelial lesions associated with HIV serostatus, CD4 cell counts, and human papillomavirus test results. JAMA 2005; 293:1471–6. [DOI] [PubMed] [Google Scholar]
- 5. Strickler HD, Burk RD, Fazzari M, et al. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus–positive women. J Natl Cancer Inst 2005; 97:577–86. [DOI] [PubMed] [Google Scholar]
- 6. Reiter PL, Pendergraft WF, 3rd, Brewer NT. Meta-analysis of human papillomavirus infection concordance. Cancer Epidemiol Biomarkers Prev 2010; 19:2916–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nyitray AG, Menezes L, Lu B, et al. Genital human papillomavirus (HPV) concordance in heterosexual couples. J Infect Dis 2012; 206:202–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bleeker MC, Hogewoning CJ, Berkhof J, et al. Concordance of specific human papillomavirus types in sex partners is more prevalent than would be expected by chance and is associated with increased viral loads. Clin Infect Dis 2005; 41:612–20. [DOI] [PubMed] [Google Scholar]
- 9. Widdice L, Ma Y, Jonte J, et al. Concordance and transmission of human papillomavirus within heterosexual couples observed over short intervals. J Infect Dis 2013; 207:1286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baken LA, Koutsky LA, Kuypers J, et al. Genital human papillomavirus infection among male and female sex partners: prevalence and type-specific concordance. J Infect Dis 1995; 171:429–32. [DOI] [PubMed] [Google Scholar]
- 11. Hippeläinen MI, Yliskoski M, Syrjänen S, et al. Low concordance of genital human papillomavirus (HPV) lesions and viral types in HPV-infected women and their male sexual partners. Sex Transm Dis 1994; 21:76–82. [DOI] [PubMed] [Google Scholar]
- 12. Thompson DL, Douglas JM, Jr, Foster M, et al. Seroepidemiology of infection with human papillomavirus 16, in men and women attending sexually transmitted disease clinics in the United States. J Infect Dis 2004; 190:1563–74. [DOI] [PubMed] [Google Scholar]
- 13. Mbulawa ZZ, Coetzee D, Marais DJ, et al. Genital human papillomavirus prevalence and human papillomavirus concordance in heterosexual couples are positively associated with human immunodeficiency virus coinfection. J Infect Dis 2009; 199:1514–24. [DOI] [PubMed] [Google Scholar]
- 14. Veldhuijzen NJ, Dhont N, Vyankandondera J, et al. Prevalence and concordance of HPV, HIV, and HSV-2 in heterosexual couples in Kigali, Rwanda. Sex Transm Dis 2012; 39:128–35. [DOI] [PubMed] [Google Scholar]
- 15. Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet 2007; 369:657–66. [DOI] [PubMed] [Google Scholar]
- 16. Tobian AA, Serwadda D, Quinn TC, et al. Male circumcision for the prevention of HSV-2 and HPV infections and syphilis. N Engl J Med 2009; 360:1298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wawer MJ, Tobian AA, Kigozi G, et al. Effect of circumcision of HIV-negative men on transmission of human papillomavirus to HIV-negative women: a randomised trial in Rakai, Uganda. Lancet 2011; 377:209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wawer MJ, Makumbi F, Kigozi G, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet 2009; 374:229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grabowski MK, Kong X, Gray RH, et al. Partner human papillomavirus viral load and incident human papillomavirus detection in heterosexual couples. J Infect Dis 2016; 213:948–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nowak RG, Gravitt PE, Morrison CS, et al. Increases in human papillomavirus detection during early HIV infection among women in Zimbabwe. J Infect Dis 2011; 203:1182–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Serwadda D, Wawer MJ, Shah KV, et al. Use of a hybrid capture assay of self-collected vaginal swabs in rural Uganda for detection of human papillomavirus. J Infect Dis 1999; 180:1316–9. [DOI] [PubMed] [Google Scholar]
- 22. Auvert B, Sobngwi-Tambekou J, Cutler E, et al. Effect of male circumcision on the prevalence of high-risk human papillomavirus in young men: results of a randomized controlled trial conducted in Orange Farm, South Africa. J Infect Dis 2009; 199:14–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Senkomago V, Backes DM, Hudgens MG, et al. Acquisition and persistence of human papillomavirus 16 (HPV-16) and HPV-18 among men with high-HPV viral load infections in a circumcision trial in Kisumu, Kenya. J Infect Dis 2015; 211:811–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Backes DM, Bleeker MC, Meijer CJ, et al. Male circumcision is associated with a lower prevalence of human papillomavirus-associated penile lesions among Kenyan men. Int J Cancer 2012; 130:1888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morris BJ, Gray RH, Castellsague X, et al. The strong protective effect of circumcision against cancer of the penis. Adv Urol 2011; 2011:812368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serwadda D, Wawer MJ, Makumbi F, et al. Circumcision of HIV-infected men: effects on high-risk human papillomavirus infections in a randomized trial in Rakai, Uganda. J Infect Dis 2010; 201:1463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tobian AA, Kacker S, Quinn TC. Male circumcision: a globally relevant but under-utilized method for the prevention of HIV and other sexually transmitted infections. Annu Rev Med 2014; 65:293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tobian AA, Kong X, Wawer MJ, et al. Circumcision of HIV-infected men and transmission of human papillomavirus to female partners: analyses of data from a randomised trial in Rakai, Uganda. Lancet Infect Dis 2011; 11:604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis MA, Gray RH, Grabowski MK, et al. Male circumcision decreases high-risk human papillomavirus viral load in female partners: a randomized trial in Rakai, Uganda. Int J Cancer 2013; 133:1247–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wilson LE, Gravitt P, Tobian AA, et al. Male circumcision reduces penile high-risk human papillomavirus viral load in a randomised clinical trial in Rakai, Uganda. Sex Transm Infect 2013; 89:262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tobian AA, Kigozi G, Gravitt PE, et al. Human papillomavirus incidence and clearance among HIV-positive and HIV-negative men in Rakai, Uganda. AIDS 2012; 26:1555–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burchell AN, Tellier PP, Hanley J, Coutlée F, Franco EL. Human papillomavirus infections among couples in new sexual relationships. Epidemiology 2010; 21:31–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gravitt PE. The known unknowns of HPV natural history. J Clin Invest 2011; 121:4593–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Weaver BA, Feng Q, Holmes KK, et al. Evaluation of genital sites and sampling techniques for detection of human papillomavirus DNA in men. J Infect Dis 2004; 189:677–85. [DOI] [PubMed] [Google Scholar]
- 35. Tobian AA, Kong X, Gravitt PE, et al. Male circumcision and anatomic sites of penile high-risk human papillomavirus in Rakai, Uganda. Int J Cancer 2011; 129:2970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]