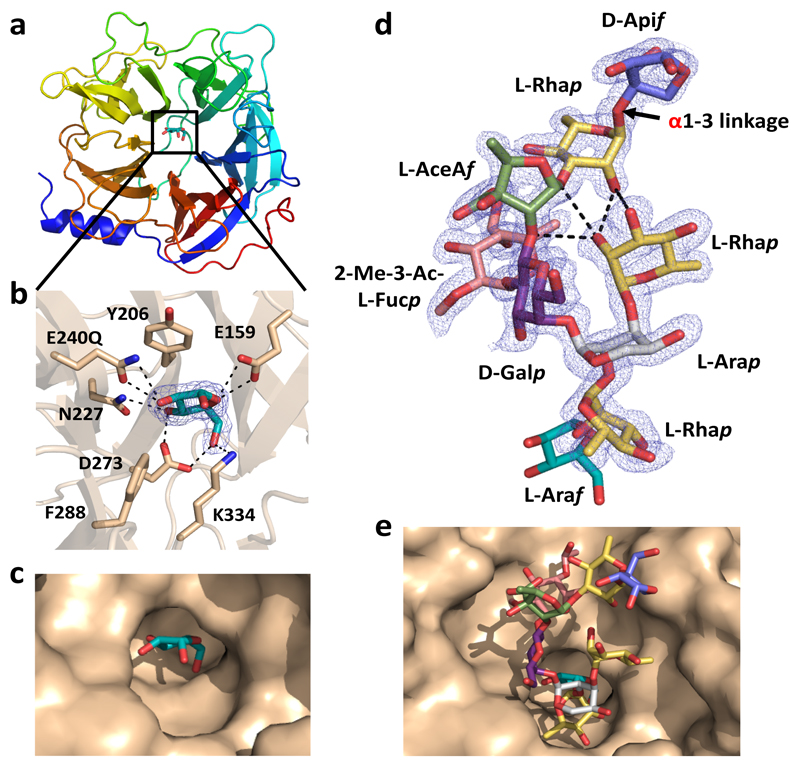
Fig. 5. Crystal structure of N-terminal catalytic domain of BT0996 (BT0996-N) in complex with RG-II-Chain B.
a, schematic of BT0996-N rainbow colour ramped from the N- (blue) to C- (red) termini. Black box; l-Araf-containing active site. b, residues interacting with l-Araf, which include the putative catalytic amino acids [Glu240 (shown as Gln240) and Glu159]. c, surface representation of the active site-pocket containing l-Araf. d, crystal structure of Chain B in complex with BT0996-N. The α linkage between l-Rhap and d-Apif is shown in red. e, shows Chain B (sugars coloured as in d) in the funnel-like substrate binding site of BT0996-N. In c,d polar interactions are broken black lines and the blue mesh is the electron density map (2Fo – Fc) of the ligands at 1.5 σ.