Figure 1.
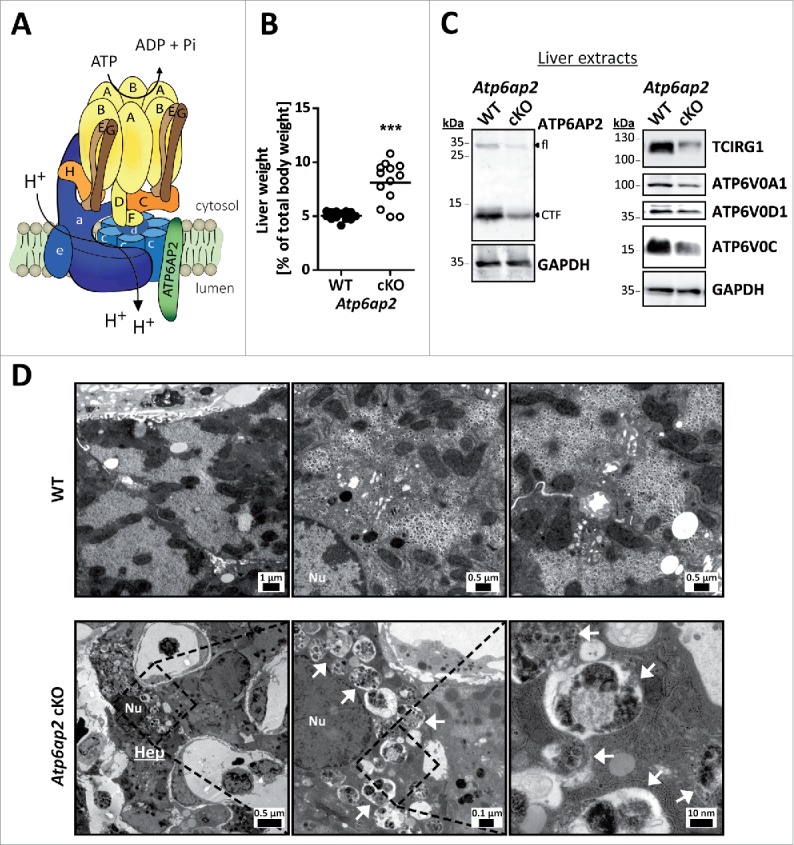
Altered ultrastructure of ATP6AP2-depleted liver. (A) Schematic illustration of the v-H+-ATPase complex composition. The v-H+-ATPase V1 sector (yellow and brown subunits, upper case letters) transfers energy released by ATP hydrolysis into a rotational force that triggers the transport of protons through the V0 sector (blue subunits, lower case letters). ATP6AP2 is an accessory subunit that acts as a chaperone for V0 sector assembly. (B) Livers from Atp6ap2 cKO mice weigh more than those of wild-type (WT) animals. (C) Immunoblot analysis of liver lysates confirms reduced concentrations of ATP6AP2 full length (fl) and C-terminal fragments (CTF) in ATP6AP2-depleted samples that come with lower v-H+-ATPase V0 sector concentrations. GAPDH detection was used as a control for equal protein load. (D) Transmission electron micrographs reveal ultrastructural changes in Atp6ap2 cKO liver as compared with wild-type samples. Affected hepatocytes (Hep) accumulate vesicles (arrows) that contain partially degraded cytoplasmic material. The boxed areas are resolved with higher magnification, Nu, nucleus.
