Figure 6. The two tryptophan residues W77 and W328 in LITE-1 are required for LITE-1 function both in vivo and in vitro.
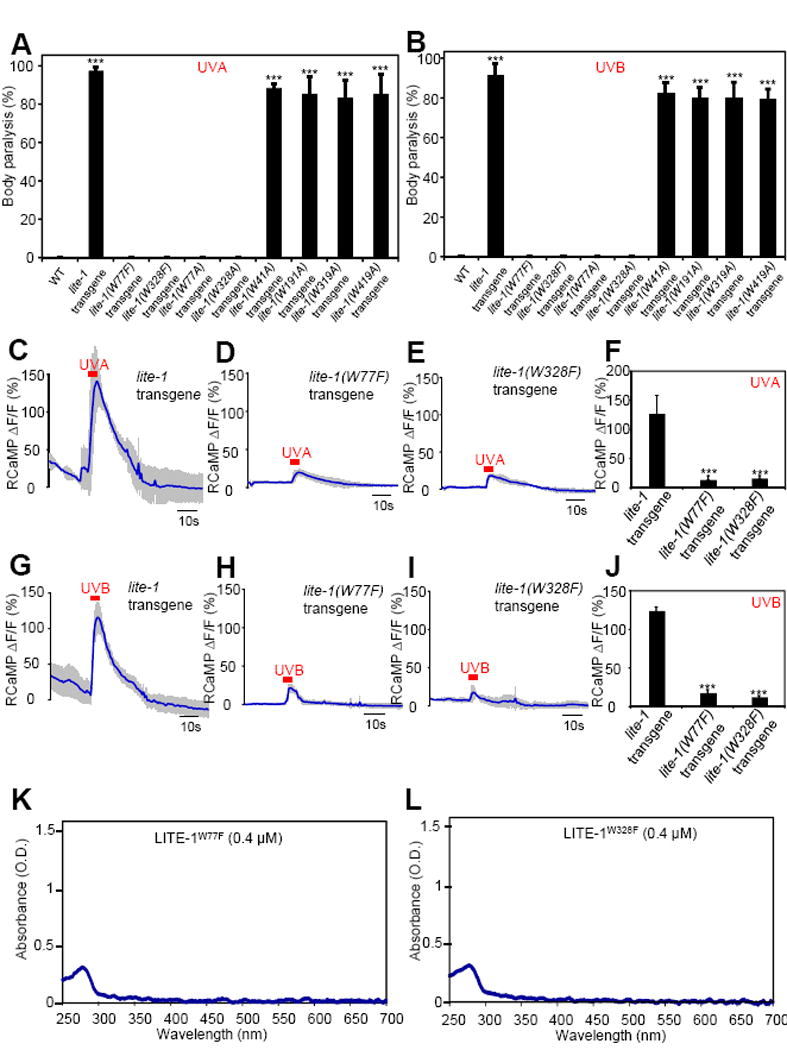
(A–B) Mutating W77 and W328 but not the other four W residues disrupts the sensitivity of LITE-1 to both UVA and UVB light in vivo shown by behavioral assays. LITE-1 variants harboring mutations in each W residue were expressed as a transgene in muscle cells. Wild-type (WT) and transgenic worms were exposed to a 20 sec pulse of UVA light (A), or UVB light (B). Animals showing muscle contraction-induced paralysis during light illumination were scored positive. n=20. Error bars: SEM. ***p<0.00001 (ANOVA with Bonferroni test).
(C–F) W77F and W328F mutations disrupt the sensitivity of LITE-1 to UVA light in vivo shown by calcium imaging. The experiments were done as described in Figure 2B. A 5 sec pulse of UVA light (340±20 nm, 0.7 mW/mm2) was applied to muscles to elicit calcium transients. Shades along the traces in (C–E) represent SEM. (F) Bar graph. n≥6. ***p<0.00001 (ANOVA with Bonferroni test).
(G–J) W77F and W328F mutations disrupt the sensitivity of LITE-1 to UVB light in vivo shown by calcium imaging. A 5 sec pulse of UVB light (280±10 nm, 0.02 mW/mm2) was applied to muscles to elicit calcium transients. Shades along the traces in (G–I) represent SEM. (J) Bar graph. n≥10. ***p<0.00001 (ANOVA with Bonferroni test).
(K–L) W77F and W328F mutations disrupt LITE-1’s absorption of both UVA and UVB light in vitro.
Also see Figure S6.
