The aim of this study was to quantify the occurrence of cardiotoxicity in a real‐life population of lymphoma patients, combining novel and traditional monitoring methods, including clinical, echocardiographic, and laboratory parameters. Comprehensive management of anthracycline‐induced cardiotoxicity consisted of a tailored use of liposomal doxorubicin in high‐risk patients, a combined monitoring approach integrated through a telemedicine system, and preemptive treatment of subclinical cardiotoxicity with beta‐blockers or renin‐angiotensin system inhibitors.
Keywords: Cardiotoxicity, Anthracyclines, Lymphoma, Non‐Hodgkin, Hodgkin Disease, Biomarkers, Doxorubicin
Abstract
Background.
Anthracyclines (AC) are still undeniable drugs in lymphoma treatment, despite occasionally causing cardiotoxicity. Liposomal AC may reduce cardiotoxicity while retaining clinical efficacy; also, biomarker monitoring during chemotherapy allows early detection of cardiac damage, enabling strategies to prevent left ventricular ejection fraction (LVEF) deterioration.
Materials and Methods.
We conducted a prospective observational trial in a real‐life population of lymphoma patients, combining advanced echocardiography and biomarkers (Troponin I [TnI]) for early detection of cardiotoxicity; we applied a prespecified policy to minimize cardiotoxicity, selecting patients with higher baseline risk to replace doxorubicin with nonpegylated liposomal doxorubicin (NPLD) and starting cardioprotective treatment when subclinical cardiotoxicity was detected.
Results.
Ninety‐nine patients received ≥1 cycle of chemotherapy (39 with NPLD): 38 (NPLD = 34) were older than 65 years. At baseline, the NPLD subgroup had more cardiovascular risk factors and comorbidities than the doxorubicin subgroup. After treatment, echocardiographic parameters did not worsen in the NPLD subgroup; significant LVEF reduction occurred in two patients treated with doxorubicin. Over treatment course, TnI rises increased linearly in the doxorubicin subgroup but modestly in the NPLD subgroup. At doxorubicin doses >200 mg/m2 the difference was statistically significant, with more TnI rises in the doxorubicin subgroup. NPLD‐treated patients did not experience higher rates of grade 3–4 adverse events. Within the diffuse large B‐cell lymphomas category, we observed similar rates of complete and overall responses between doxorubicin‐ and NPLD‐treated patients.
Conclusion.
A comprehensive strategy to prevent, detect, and treat cardiotoxicity allows an optimal management of the lymphoma with low incidence of cardiac complications.
Implications for Practice.
Despite the recent advances of targeted therapy in cancer, old cytotoxic drugs such as anthracyclines (AC) still play a fundamental role in the treatment of many lymphoma patients. We tested and validated in a real‐life setting a personalized approach to prevent, detect, and treat AC‐induced cardiotoxicity; biomarker monitoring was accomplished by Troponin I measurements before and after chemotherapy infusions, allowing detection of early subclinical cardiotoxicity, which was preemptively treated with cardio‐protectants (beta blockers and angiotensin‐converting‐enzyme inhibitors). A telemedicine system allowed interdisciplinary management of the patients with an expert cardiologist. Furthermore, tailored use of liposomal AC following a prespecified policy appeared to prevent the excess cardiotoxicity expected in high‐risk patients.
Introduction
Anthracyclines (AC) are the mainstay of first‐line treatment in many lymphoma patients. A specific concern of AC‐containing regimens is the occurrence of cardiac toxicity [1]. Although cardiac adverse effects of AC were recognized shortly after their discovery, still basic aspects related to management of AC‐induced cardiotoxicity (AIC) remain unclear [2], [3]. First, the prevalence of AIC is highly variable in different settings, but only age and cumulative AC dose are consistently recognized as relevant risk factors [4]. Further uncertainty is added in the hematological setting, as most recommendations are inferred by studies conducted in solid tumors, primarily breast and lung cancer [5], [6] The exact prevalence of AIC, occurring after widely used regimens such as R‐CHOP for non‐Hodgkin lymphomas (NHL) or ABVD for Hodgkin's disease (HD), is still unknown in the real life: in fact, the large clinical studies that tested these interventions were mainly focused on efficacy. Second, several monitoring methods have been proposed, but there is no clear indication yet about the best technique, although echocardiogram is generally preferred due to ease of use [7]. Notably, the sensitivity of the detection method will also impact the reported prevalence of AIC. Thus, the only population‐based study addressing AIC in lymphoma inherently lacks sensitivity to detect subclinical AIC [8].
Several strategies have been suggested to reduce AIC without negatively affecting antitumor efficacy. The most applied of these strategies is limitation of the cumulative dose according to current prescribing recommendations [9]; also, weekly or continuous infusion protocols have been shown to be equally effective but less toxic than classic scheduling every 2 or 3 weeks; however such protocols have limited applicability in outpatient settings [10]. The cardioprotectant dexrazoxane has been shown to decrease AIC when administered concomitantly with AC. However, concerns of reduced antitumor activity and a reported increase in secondary tumors in pediatric populations discouraged dexrazoxane use in clinical practice [11], [12]. Liposomal formulations of doxorubicin offer pharmacokinetic advantages over the free drug [13]; nonpegylated liposomal doxorubicin (NPLD) showed reduced AIC with preserved antitumor efficacy in metastatic breast cancer [14]. These data prompted use in lymphoma patients: several single‐arm trials suggested an encouraging efficacy and reduced incidence of AIC, although we lack a large randomized comparison with conventional AC [15], [16].
A major advance in the management of heart failure was the recognition and preemptive treatment of subclinical structural heart damage to prevent further deterioration of cardiac function leading to symptomatic heart failure [17]. Similarly to heart failure from other causes, signs of cardiac dysfunction may be detected a long time before clinical manifestations of AIC [18]. Specifically, a monitoring strategy including serial measurements of cardiac troponins may identify the patients at very high risk for AIC in their earliest phase [19]. This observation supports the existence of a temporal window that may be exploited by therapeutic interventions aiming to stop and reverse the cardiac damage.
The aim of our study was to quantify the occurrence of cardiotoxicity in a real‐life population of lymphoma patients, combining novel and traditional monitoring methods, including clinical, echocardiographic, and laboratory parameters. To improve the feasibility of such a combined monitoring approach in an outpatient setting, we tested the performance of a telemedicine system to integrate data and to allow constant interdisciplinary management with an expert cardiologist. Regarding biomarkers, we aimed to evaluate the occurrence and kinetics of Troponin I (TnI) rises and their correlation to clinical and subclinical cardiotoxicity.
Subjects, Materials, and Methods
This is a prospective observational trial in lymphoma patients undergoing first‐line treatment with conventional or liposomal AC at our center (Hematology Clinic, Ancona, Italy). Inclusion criteria were age greater than 18 years, diagnosis of lymphoma according to WHO classification (2008), and planned treatment with a chemotherapy protocol containing conventional or liposomal AC. Exclusion criteria were lack of informed consent, previous treatment with chemotherapy or radiotherapy (with the exception of high‐dose corticosteroids administered for less than 30 days), and fertile women unwilling to use contraception.
The study was approved by our institutional review board and registered in the Agenzia Italiana del Farmaco registry of observational studies (Study ID: RSO‐581). All patients provided informed consent. Patients were enrolled from February 2012 to March 2014. The primary objective of the study was to determine the incidence rate of AIC, which was defined as a significant drop in LVEF as in Swain et al. [6] (>10% to a final value <50%; >20%; or drop to a final value <45%, irrespective of the initial value), detected at any time during treatment until 1 year after treatment completion. The secondary objectives were to determine (a) the incidence of cardiac adverse effects possibly related to AIC (sudden death, acute heart failure, arrhythmia requiring treatment, acute myocardial infarction or unstable angina, worsening of New York Heart Association class, symptomatic pericarditis); (b) the occurrence of significant echocardiographic abnormalities other than LVEF reduction (increase in size of cardiac chambers and walls, valvulopathies, pericardial diseases); (c) the occurrence of TnI rises (above the prespecified cut‐offs of 0.03 ng/mL and 0.08 ng/mL) not related to ischemic events; (d) the feasibility of a combined monitoring approach using a telemedicine system; (e) the response to the chemotherapy treatment (according to 2007 International Working Group criteria [20]); and (f) overall survival (OS).
Chemotherapy treatments for lymphomas were administered according to current standard protocols (supplemental online appendix); the only types of AC used were doxorubicin (DOX) and NPLD hydrochloride (Myocet, Teva) (NPLD). Substitution of DOX with NPLD at equivalent doses was decided according to our internal policy, reported in Table 1. Cardiologic assessments were performed at least every 100 mg/m2 of doxorubicin cumulative dose with a telemedicine system, which was developed as an electronic platform for cardiology consultations. Each assessment included ECG and echocardiography and allowed for therapeutic suggestions when an alteration was detected. TnI measurements were performed before, 1 hour after, and 24–72 hours after each chemotherapy cycle. Detection of TnI rises above 0.08 ng/mL prompted start of cardioprotective treatment (angiotensin‐converting‐enzyme [ACE] inhibitors, angiotensin receptor blockers, or beta blockers at low doses, according to the cardiologist). Details of cardiac assessment, concomitant treatments, and statistical analyses are reported in the supplemental online Appendix.
Table 1. Stratification and treatment allocation according to our prespecified internal policy (filed in October 2011 with the local health administration).
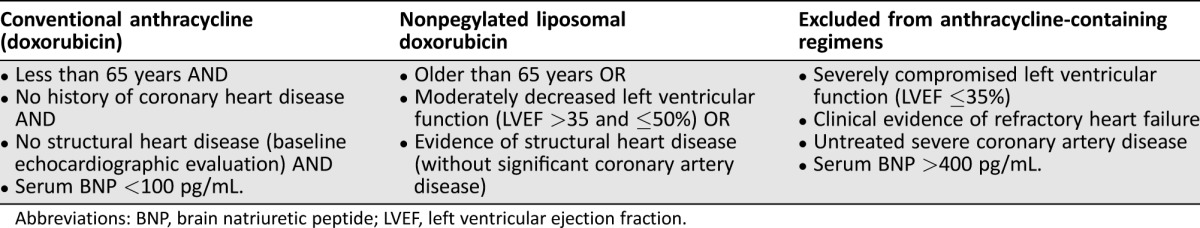
Abbreviations: BNP, brain natriuretic peptide; LVEF, left ventricular ejection fraction.
Results
Baseline Characteristics and Planned Treatments
We enrolled a total of 104 patients, of whom 5 never started therapy at our center: 3 died before starting treatment and 2 were treated in another hematology center due to logistic preferences. Data are reported for the remaining 99 patients who underwent at least 1 cycle of chemotherapy (modified intention‐to‐treat population).
The baseline characteristics of the 99 patients are shown in Table 2. The median age was 60 years (range 18–85 years), and 38 patients were older than 65 years. Twenty‐five had HD and, 74 had NHL. The most represented NHL subtype was diffuse large B‐cell lymphoma (DLBCL) in 53% of patients. All HD patients were treated with the ABVD protocol, with NPLD substituting for DOX in six patients. All 74 NHL patients were treated with CHOP‐like regimens, with NPLD substituting for DOX in 33 patients. Etoposide was added for T‐cell lymphomas; in the 70 B‐cell NHL, the anti‐CD20 antibodies rituximab (69 patients) or obinutuzumab (1 patient) were added. Overall, NPLD was used in 39 patients: 35 were older than 65 years, and 4 were younger than 65 years but had evidence of structural heart disease or significant past history of heart diseases. Of 38 patients older than 65 years, 3 were treated with DOX, contradicting our internal policy: substitution with NPLD was not possible, as they were enrolled in interventional trials not allowing the use of NPLD; they all had excellent performance status and no comorbidities. The average cumulative AC dose was 262 mg/m2 (268mg/m2 for DOX‐treated patients and 254 mg/m2 for NPLD‐treated patients).
Table 2. Baseline characteristics of the 99 patients undergoing at least 1 cycle of CHT, related to demographics, disease characteristics and planned treatments.
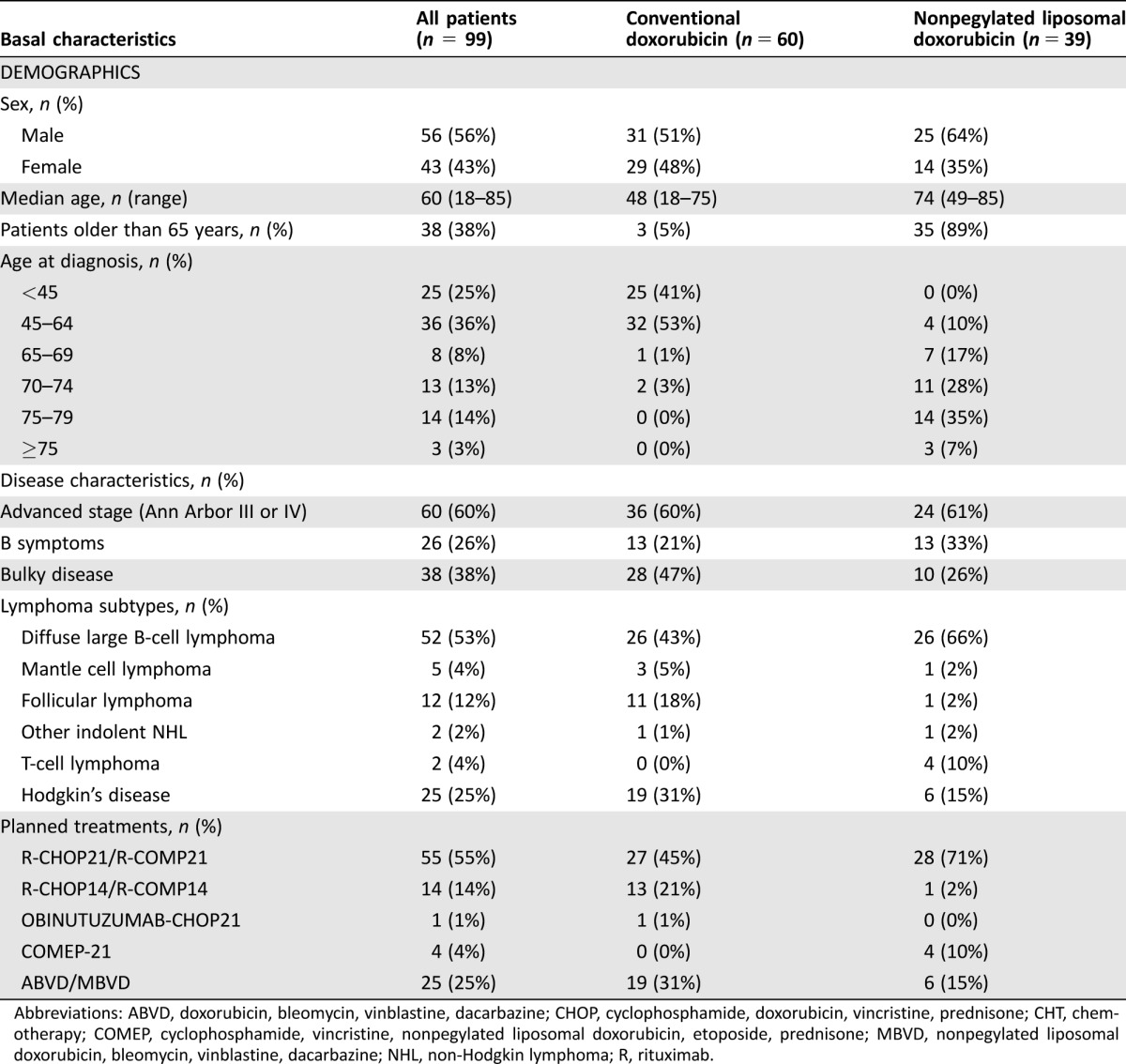
Abbreviations: ABVD, doxorubicin, bleomycin, vinblastine, dacarbazine; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CHT, chemotherapy; COMEP, cyclophosphamide, vincristine, nonpegylated liposomal doxorubicin, etoposide, prednisone; MBVD, nonpegylated liposomal doxorubicin, bleomycin, vinblastine, dacarbazine; NHL, non‐Hodgkin lymphoma; R, rituximab.
Over half the patients (57%) had at least one of the following traditional cardiac risk factors (Table 3), excluding age > 60 years: obesity (body mass index >30 kg/m2, n = 5), diabetes mellitus (n = 6), hypertension (n = 29), hyperlipidemia (n = 18), history of tobacco use (n = 28), and/or family history of heart disease (n = 6). Other important comorbidities that increase the risk of developing heart failure included a history of coronary artery disease (CAD, n = 8), atrial fibrillation (n = 5), and a history of hypothyroidism (n = 7). At baseline, 25% of patients were receiving either renin‐angiotensin system (RAS) inhibitors or beta blockers (Table 3).
Table 3. Baseline characteristics of the 99 patients undergoing at least 1 cycle of CHT, related to cardiac risk factors and comorbidities, laboratory values and cardiac evaluation before treatment.
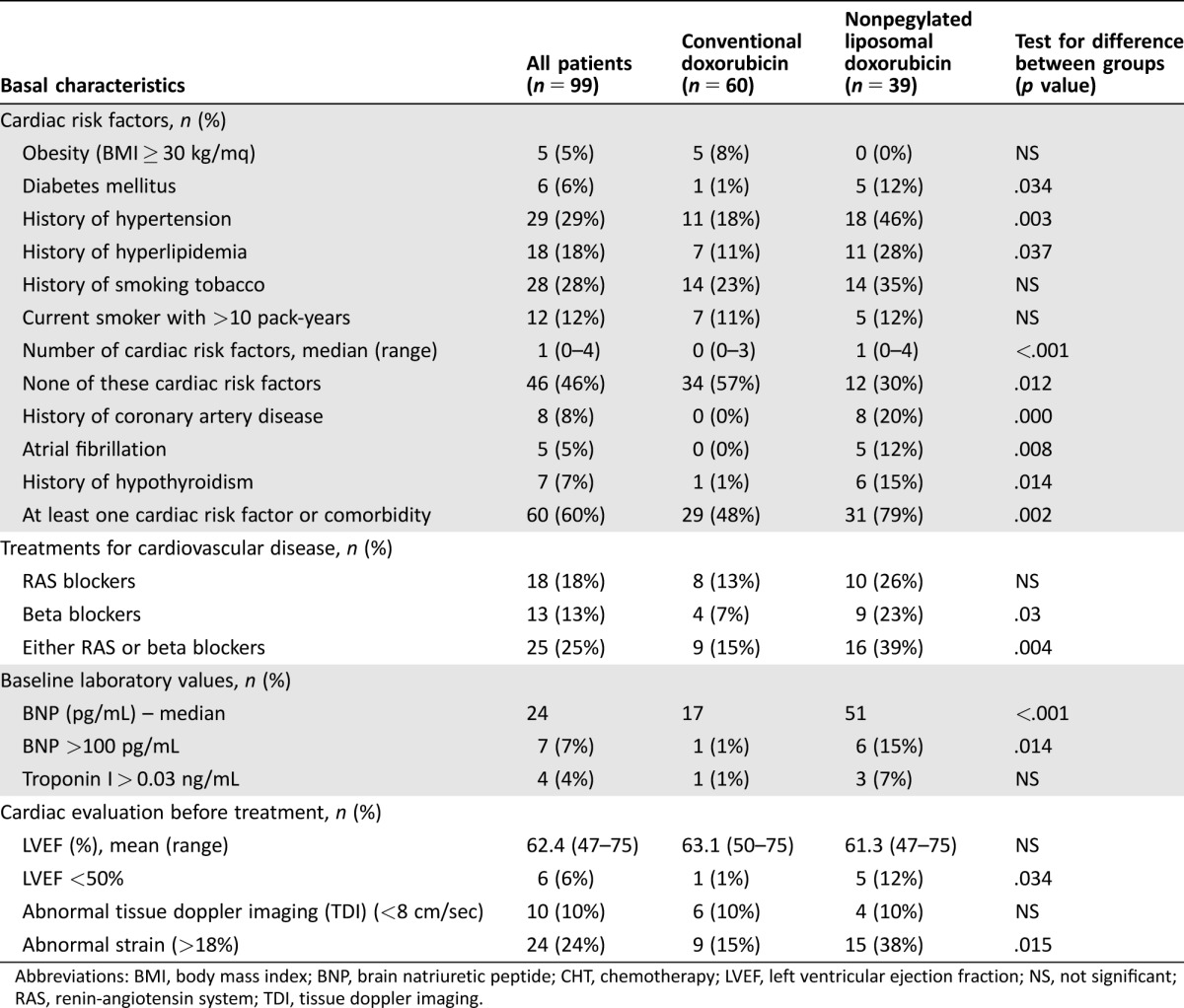
Abbreviations: BMI, body mass index; BNP, brain natriuretic peptide; CHT, chemotherapy; LVEF, left ventricular ejection fraction; NS, not significant; RAS, renin‐angiotensin system; TDI, tissue doppler imaging.
The distribution of cardiac risk factors and comorbidities showed obvious imbalances across the two groups, as expected by the categorization recommended by our internal policy. Overall, the subgroup of patients treated with NPLD was significantly older than the subgroup treated with DOX (mean age 72.3 and 46.1 years in NPLD and DOX subgroups, respectively; p < .001); they had significantly more risk factors for heart disease (median number 1 and 0 in NPLD and DOX subgroups, respectively; p < .001); in detail, in the NPLD subgroup, there was increased prevalence of diabetes mellitus, hypertension, hyperlipidemia, but not of obesity or tobacco use. Eight patients reported previous history of CAD; of these, three had concurrent atrial fibrillation and two more patients had atrial fibrillation without previous history of CAD; according to our internal policy, all these patients were treated with NPLD. Family history of premature heart disease was reported in five patients (8.3%) treated with DOX and one patient (2.6%) treated with nonpegylated liposomal doxorubicin (NPLD) (p = NS); more patients in the NPLD subgroup had missing, incomplete, or unreliable information (26% and 13% in the NPLD and DOX subgroups, respectively), perhaps due to the different ages of the two groups.
Use of RAS or beta blockers in DOX and NPLD subgroups (18% and 46%, respectively; p = .004) grossly mirrored the prevalence of hypertension. Two hypertensive patients (18%) in the DOX subgroup and five (28%) in the NPLD subgroup were not receiving RAS or beta blockers; they were either receiving no treatment or other treatments (e.g., calcium‐channel inhibitors, diuretics). Three patients in the NPLD subgroup and none in the DOX subgroup were receiving RAS or beta blockers for indications other than hypertension.
Echocardiographic evaluation at study entry did not reveal significant differences between NPLD and DOX subgroups (mean LVEF at baseline in all patients and NPLD and DOX subgroups was 62.4%, 61.3%, and 63.1%, respectively). Three patients had baseline LVEF lower than 50%; they were all treated with NPLD. Patients in the NPLD subgroup had higher frequency of abnormal global longitudinal strain (≥−18%, n = 15 in NPLD subgroup; n = 9 in DOX subgroup, p = .015) but not of abnormal tissue doppler imaging (TDI).
As regards biomarker evaluation at baseline, the brain natriuretic peptide (BNP) was above the upper normal limit (100 pg/mL) in seven patients: six and one in the NPLD and DOX subgroups, respectively (p < .001); only one patient in the NPLD subgroup had a baseline TnI value above the upper normal limit (0.08 ng/mL). Four patients had a baseline TnI above 0.03 ng/mL, three and one in the NPLD and DOX subgroups, respectively (p = NS).
Treatment Delivery, Main Toxicities, and Outcome
Of the 99 patients receiving ≥1 cycle of chemotherapy, 14 (10 treated with NPLD) did not complete the planned induction treatment: 7 (6 in the NPLD subgroup) died during treatment, 5 (4 in the NPLD subgroup) had unbearable toxicity, and 2 patients treated with DOX showed insufficient response at planned interim restaging, which prompted treatment change.
Out of 566 cycles of chemotherapy planned, 518 were actually administered (91.5%). Relative dose intensity was above 99% for most of the chemotherapy drugs employed (bleomycin, cyclophosphamide, dacarbazine, DOX, etoposide, NPLD, obinutuzumab, rituximab) either in the DOX or NPLD subgroups. The only exceptions were vinblastine, which was administered at 98% and 96% of the planned dose in DOX and NPLD subgroups, respectively, and vincristine, which was administered at 95% and 89% of the planned dose in DOX and NPLD subgroups, respectively.
The various treatments administered did not result in a burden of toxicities substantially different from historical cohorts. The grade 3 or greater toxicities, including both cardiac and noncardiac events observed during treatment, are summarized in Table 4. Overall, there were no significant differences in the rates of grade ≥3 adverse events between the DOX and NPLD subgroup.
Table 4. Selected hematological and extra‐hematological adverse events occurring during treatment in the 99 patients undergoing at least 1 cycle of CHTa.

Numbers outside parentheses represent the proportion of all patients in the cohort (N=99) developing adverse events; the percentage reported inside parentheses refers to the occurrence of adverse events in the cohort of patients treated with liposomal doxorubicin (N = 39).
Abbreviations: CHT, chemotherapy; DLBCL, diffuse large B‐cell lymphoma.
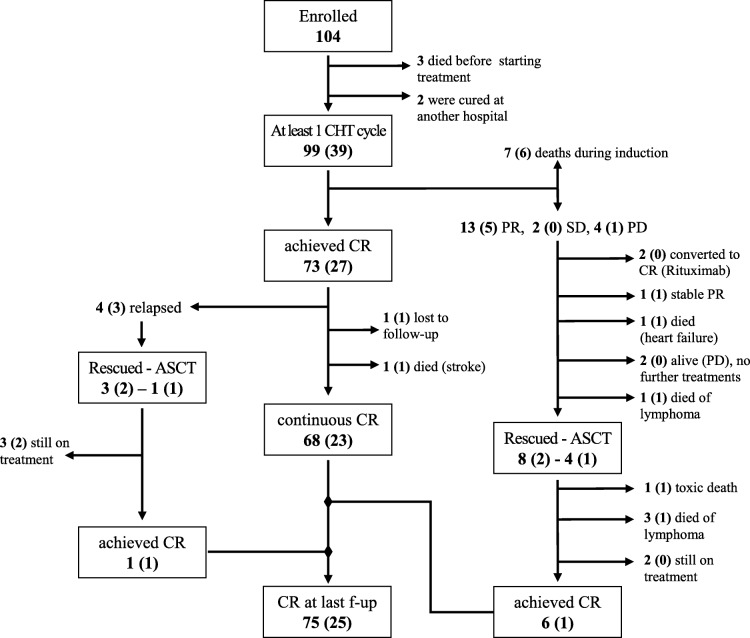
At the end of induction treatment, 92 patients (33 treated with NPLD) were evaluable for response: complete response (CR) was achieved in 79% (n = 73, of which n = 23 treated with NPLD); partial response (PR) in 14% (n = 13, of which n = 5 treated with NPLD); stable (SD) or progressive disease (PD) in 7% (n = 6, of which n = 1 treated with NPLD). The corresponding response rates for DLBCL, which represented the largest disease category (n = 48 evaluable for response, of which 23 treated with NPLD), are CR in 83% of patients (87% in the NPLD subgroup), PR in 13% (13% in the NPLD subgroup), and PD in 4% (no patients in the NPLD subgroup). At last follow‐up, 76 patients (24 treated with NPLD) are alive and in CR. Detailed outcomes about further lines of treatment are reported in Figure 1.
Figure 1.
Study flow‐chart describing the outcome of patients. The corresponding figures for NPLD patients are shown in parentheses.
Abbreviations: ASCT, autologous stem cell transplantation; CHT, chemotherapy; CR, complete response; PD, progressive disease; PR, partial response; SD, stable disease.
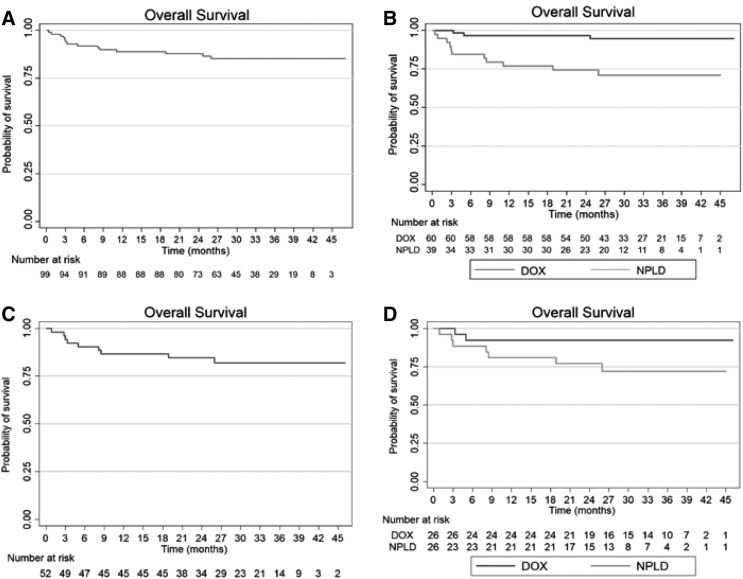
With a median follow‐up of 31.7 months, the estimated probability of OS at 3 years is 85.3% in the entire cohort (Fig. 2); in the DOX and NPLD subgroups, the corresponding rates are 94.7% and 71.0%, respectively (p < .001). Restricting the analysis to patients with DLBCL, the 3‐year OS is 81.8% in the entire cohort and 92.3% and 71.8% in the DOX and NPLD subgroups, respectively (p = .08).
Figure 2.
Overall survival of the entire cohort of patients (A) and overall survival according to type of anthracycline (B), as well as overall survival in the subgroup of patients with DLBCL in the entire cohort (C) and according to type of anthracycline (D).
Abbreviations: DOX, doxorubicin; NPLD, nonpegylated liposomal doxorubicin.
Overall, 14 patients have died: 7 during induction treatment and 7 thereafter. Causes of death were disease progression in five patients (two treated with NPLD), cerebrovascular accidents in two patients treated with NPLD, treatment‐related in four patients treated with NPLD, and cardiac disease in three patients treated with NPLD (detailed in the supplemental online Appendix): all three of these patients had severe preexisting cardiovascular comorbidities, and deaths were not clearly attributable to AIC.
Cardiac Toxicity: Echocardiographic Evaluation
The primary endpoint of the study (reduced LVEF >10% to a final value <50%), was evaluable in in 85 patients (28 treated with NPLD). Of the remaining 14 patients, 7 died during induction treatment, 3 died shortly after completing induction treatment, and 4 were unavailable to recall evaluations due to logistic difficulties; however, at last follow‐up, they did not report significant cardiac events, and recent cardiac evaluations were normal. Two patients treated with DOX (2.4%) had LVEF reductions meeting the criteria for the primary endpoint. In both cases, prompt initiation of treatment with ACE inhibitors or beta blockers lead to complete reversal of LVEF and other cardiac abnormalities (supplemental online Appendix).
Five other patients developed other cardiac abnormalities without a significant LVEF reduction. In three patients (one treated with NPLD), we found appearance or mild worsening of valvular diseases; one patient treated with DOX developed mild enlargement of the left ventricle; one patient treated with NPLD had progressive worsening of a preexisting right ventricular dysfunction, which ultimately led to death.
The mean LVEF change from the baseline in the entire cohort was −1.25% (SD = 6.67, range −18 to +18; t test for comparison between baseline and follow‐up values: p = .09); it did not differ in the patients treated with DOX (−1.61%, SD = 6.61, range −16 to +14) or NPLD (−0.5%, SD 6.86, range −18 to +18; t test for comparison between DOX and NPLD: p = .47). Overall, eight patients had absolute LVEF reductions greater than 10%: seven in the DOX subgroup (including the two patients meeting the primary endpoint) and one in the NPLD subgroup.
A complete echocardiographic evaluation, including measurements of global strain rate (GSR) and tissue Doppler velocity performed both at baseline and at follow‐up, was available for 73 patients (50 and 23 in the DOX and NPLD subgroups, respectively). There was a significant increase of the GSR (meaning reduced ventricular function) from the baseline in the entire cohort (mean change +1.2%, SD = 3.2, range −8.9 to +9.2, p = .003). There was also a trend toward higher increases in the patients treated with DOX (+1.61%, SD = 2.8, range −8.9 to +7.3) with respect to patients treated with NPLD (−0.6%, SD 3.7, range −7.4 to +9.2; t test for comparison between DOX and NPLD: p = .05).
As regards TDI evaluation, there was a significant decrease from baseline to follow‐up values in the entire cohort (mean change −12.1 cm/sec, SD = 20.8, range −70 to +30; t test for comparison between baseline and follow‐up values: p < .001). TDI reduction did not differ in the patients treated with DOX (−13.7%, SD = 21.6, range −70 to +30) or NPLD (−8.6%, SD = 19.1, range −60 to +30; t test for comparison between DOX and NPLD: p = .35).
Cardiac Toxicity: Biomarker Evaluation
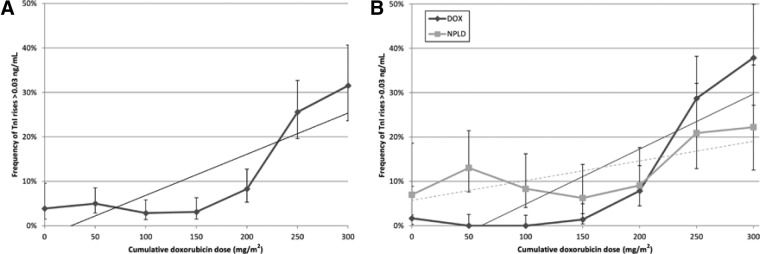
We performed a total of 1,307 TnI measurements. Only 1.8% of TnI measurements resulted above 0.08 ng/mL; 9.6% resulted above 0.03 ng/mL. TnI increases occurred more frequently at cumulative doxorubicin doses ≥200 mg/m2 considering both the cut‐off of 0.03 ng/mL (3.6% and 20.7% at doses < and ≥200 mg/m2, respectively, p < .001) and of 0.08 ng/mL (1.2% and 3.0% at doses < and ≥200 mg/m2, respectively, p < .029); also, the frequency of TnI rises above 0.03 ng/mL had excellent linear correlation with the cumulative dose of doxorubicin (Fig. 3A; Pearson's rho = 0.84; p = .019).
Figure 3.
Kinetics of the TnI rises at increasing cumulative doxorubicin doses in all patients (A) and according to anthracycline type (B). At cumulative doxorubicin doses <200 mg/m2, TnI rises above 0.03 ng/mL occur more frequently in the NPLD subgroup (9.3% and 0.6% in the NPLD and DOX subgroups, respectively; p < .001); however, at doses >200 mg/m2, the relationship is reversed, with higher frequency in the DOX subgroup (16.0% and 21.4% in the NPLD and DOX subgroups, respectively: p = .047).
Abbreviations: DOX, doxorubicin; NPLD, nonpegylated liposomal doxorubicin; TnI, Troponin I.
Thirteen patients (13%) developed a TnI increase above 0.08 ng/mL, and 42 patients (42%) developed a TnI increase above 0.03 ng/mL. In two patients, the TnI rises reflected the occurrence of an asymptomatic acute coronary syndrome, detected by the telemedicine system (supplemental online Appendix). Overall, TnI increases above 0.03 ng/mL occurred more frequently in patients treated with NPLD (11.9% and 8.3% in the NPLD and DOX subgroups, respectively; p = .034).
In DOX‐treated patients we could observe the same excellent linear correlation between TnI rises above 0.03 ng/mL and the cumulative doxorubicin dose (Pearson's rho = 0.86; p = .013). In the NPLD subgroup, the frequency of TnI rises above 0.03 ng/mL no longer retained this correlation (Fig. 3B; Pearson's rho = 0.69; p = .084). At cumulative doxorubicin doses ≤200 mg/m2, TnI rises above 0.03 ng/mL occurred more frequently in the NPLD subgroup (9.3% and 0.6% in the NPLD and DOX subgroups, respectively; p < .001); at doses >200 mg/m2, the relationship was reversed, with higher frequency in the DOX subgroup (16.0% and 21.4% in the NPLD and DOX subgroups, respectively: p = .047).
Discussion
This prospective observational trial was aimed to an early detection of AIC in a real‐life population of lymphoma patients receiving conventional (DOX) or liposomal AC (NPLD). Basing on a prespecified internal policy, patients with higher risk factors for AIC received treatment with NPLD. As expected, the group of patients treated with NPLD was older and had more cardiac risk factors compared with the DOX‐treated group. Cardiac evaluation for AIC at baseline and follow‐up employed both advanced echocardiographic techniques and biomarkers (TnI). Overall, we found a low incidence of significative LVEF reduction approximately 1 year after ending treatment (all cases in the DOX group); the mean LVEF of the entire cohort was not significantly reduced, but other echocardiographic parameters (GSR, TDI) showed a mild impairment after chemotherapy. Also, we observed more frequent TnI rises with increasing cumulative doses of AC. When comparing the two treatment groups at baseline, we found that the echocardiographic evaluation did not differ for LVEF and TDI, but GSR values were worse in the NPLD group; however, after chemotherapy, the NPLD group had less impairment in GSR than the DOX group. Such trends were even more evident for the biomarker evaluation: the DOX group started treatment with a low frequency of TnI rises and developed an exponential increase; in the NPLD group, TnI rises were quite frequent at baseline but had only a limited increase during treatment.
Despite the recent advances of targeted therapy in cancer, it is not yet time to mothball chemotherapy in lymphoma. This is particularly true in the setting of DLBCL, in which AC in the front‐line seems undeniable and experimental protocols are trying to improve the outcomes by adding novel drugs to the old reliable CHOP backbone and not by substituting it [1], [21]. The improvement in cancer therapeutics has also led to an apparent paradox in some situations: in HD, treatment‐related deaths are exceeding those related to the underlying disease [22]; therefore, chemotherapy regimens have been refined, aiming for the minimum amount of therapy needed to preserve the antitumor efficacy. However, in the era of personalized medicine, novel strategies are needed to reduce toxicity beyond a flat reduction in the drug dose. With respect to AIC, today we have improved diagnostic techniques and new cardioprotective strategies, but the approach in the clinical practice has remained quite the same in the last 40 years.
In our study, we sought to take advantage of the recent technical, pharmacological, and clinical advances in the field of cardiotoxicity to build a comprehensive strategy aimed to prevent, detect, and treat AIC. Recently, routine monitoring of TnI during chemotherapy has shown to detect cardiotoxicity in its earliest phase, long before any reduction in LVEF has occurred [23], [24]. In those patients with a TnI rise, starting a cardioprotective treatment with ACE inhibitors (enalapril) may prevent LVEF deterioration and other cardiac events [25]. The predictive value of TnI for AIC has been confirmed in various studies [26]. Moreover, monitoring with TnI during treatment with AC has been recently recommended by the guidelines of the European Society of Medical Oncology to prevent from chemotherapy‐induced cardiotoxicity [27].
Advanced echocardiographic techniques are currently explored to improve detection of subclinical signs of impaired ventricular function that would be missed by conventional echocardiography [7]. Such novel approaches are under investigation also for chemotherapy‐induced cardiotoxicity: a recent systematic review focused on the role of strain rate imaging for the early detection of myocardial changes and prediction of cardiotoxicity in patients receiving cancer therapy. The study found that, in late survivors of cancer, measures of global radial and circumferential strain are consistently abnormal, even in the context of normal LVEF; however, the authors concluded that the clinical value of global strain imaging in predicting subsequent ventricular dysfunction or heart failure has not been established [28].
The availability of new liposomal formulations provides the opportunity to improve the therapeutic window of AC, but to this end, the lymphoma setting has yet to be explored. In Italy, NPLD is currently included in the treatment of aggressive NHL in elderly patients or in those with cardiac comorbidities (according to an extension of the approved indication [29]); however, there are as yet no study confronting conventional and liposomal AC in this setting of patients. Recently, an Austrian multicenter study reported a randomized comparison between R‐CHOP and rituximab, cyclophosphamide, vincristine, nonpegylated liposomal doxorubicin, predniso (R‐COMP) in 79 adult patients with DLBCL, but the investigation included a selected population without cardiac risk factors [30]. The study did not find a significant difference in the primary end point (i.e., LVEF values 4–8 weeks after the end of treatment) between the two groups.
To our knowledge, our study is the first reporting a prospective comparison between conventional and liposomal AC in a real‐life setting of lymphoma patients. Not being a randomized study, we could observe obvious imbalances in the baseline distribution of cardiac risk factors and comorbidities: the NPLD subgroup was unquestionably older and sicker than the DOX subgroup. Such inequality was intentional, as it served to select patients at high risk for AIC in order to allocate them to the putative less toxic treatment. Nevertheless, regarding cardiotoxic events, we did not observe an increased incidence of negative outcomes for the NPLD subgroup. On the contrary, the GSR evaluated after the end of treatment was significantly more impaired in the DOX‐treated patients with respect to the NPLD subgroup. The biomarkers data further suggest that NPLD may limit subclinical damage in patients at high risk for AIC: indeed, an exponential increase in TnI alterations was noted in the DOX subgroup but not in the NPLD subgroup. Our results are in line with those reported by the Austrian randomized trial, showing a worsening biomarker profile (evaluated by serial BNP measurements) with higher AC cumulative doses in the R‐CHOP arm; on the contrary, BNP values remained flat over the treatment course in the R‐COMP arm [30].
Cardioprotective treatment with ACE inhibitors or beta blockers is another appealing strategy to reduce AIC in high‐risk patients: in our algorithm, we started secondary cardioprotective treatment only after detection of subclinical AIC. However, more patients in the NPLD subgroup were already on RAS or beta blockers at baseline due to higher prevalence of cardiac comorbidities in this subgroup. Effective treatment of cardiovascular diseases can no doubt prevent their negative effects and thus may contribute to the protective effect seen in the NPLD subgroup; furthermore, benefits may hold when such drugs are used in primary prevention. Bosch et al. [31] randomized 90 patients without left ventricular systolic dysfunction who submitted to intensive chemotherapy for hematological malignancies to combined therapy with enalapril and carvedilol or no intervention and found that primary cardioprotective treatment reduced chemotherapy‐induced LVEF deterioration. Such strategy appears promising and deserves further investigation in larger trials.
Regarding the efficacy evaluation, our study has the obvious limitation of including several lymphoma subtypes. However, among DLBCL, we observed similar rates of CR in DOX and NPLD subgroups, although baseline international prognostic index (IPI) was significantly higher in the NPLD subgroup (p = .049), reflecting the older age of these patients. The NPLD subgroup had a trend toward lower OS (log‐rank p = .08), primarily owing to early treatment‐related deaths, which remain a significant concern in elderly DLBCL patients [32]. To this end, other strategies such as a prephase treatment [33] or tailored dose reduction [34] may be combined with NPLD use in order to reduce toxicity in frail patients.
Conclusion
We aimed to measure the prevalence of AIC in a setting of lymphoma patients in which AC still represents the mainstay of the first‐line treatment. We observed a very low incidence of AIC (2.4%) with respect to historical comparisons [35]. A possible explanation is a spurious effect due to the relatively small sample size of our study; however, it is plausible that a comprehensive strategy including tailored NPLD use, multimodal monitoring, and prompt initiation of a cardioprotective treatment may have reduced the occurrence of AIC. Finally, our study suggests that DOX substitution with NPLD may be profitable in lymphoma patients at high risk for AIC because it prevented the excess of cardiac toxicity expected while preserving the antitumor efficacy.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank the Associazione Italiana contro le Leucemie (AIL) for broadly supporting our research.
Author Contributions
Conception/Design: Jacopo Olivieri, Gian Piero Perna, Attilio Olivieri, Guido Gini
Provision of study material or patients: Jacopo Olivieri, Gian Piero Perna, Caterina Bocci, Claudia Montevecchi, Guido Gini
Collection and/or assembly of data: Jacopo Olivieri
Data analysis and interpretation: Jacopo Olivieri
Manuscript writing: Jacopo Olivieri
Final approval of manuscript: Jacopo Olivieri, Attilio Olivieri, Pietro Leoni
Disclosures
Jacopo Olivieri: Teva Italia (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supplementary Information
References
- 1. Luminari S, Montanini A, Federico M. Anthracyclines: A cornerstone in the management of non‐Hodgkin's lymphoma. Hematol Rep 2011;3(3s):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Marco A, Gaetani M, Scarpinato B. Adriamycin (NSC‐123,127): A new antibiotic with antitumor activity. Cancer Chemother Rep 1969;53:33–37. [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Survivorship version 2.2015. Available at http://www.nccn.org/professionals/physician_gls/pdf/survivorship.pdf. Accessed November 7, 2015.
- 4. Smith LA, Cornelius VR, Plummer CJ et al. Cardiotoxicity of anthracycline agents for the treatment of cancer: Systematic review and meta‐analysis of randomised controlled trials. BMC Cancer 2010;10:337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Von Hoff DD, Layard MW, Basa P et al. Risk factors for doxorubicin‐induced congestive heart failure. Ann Intern Med 1979;91:710–717. [DOI] [PubMed] [Google Scholar]
- 6. Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003;97:2869–2879. [DOI] [PubMed] [Google Scholar]
- 7. Plana JC, Galderisi M, Barac A et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: A report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2014;15:1063–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hershman DL, McBride RB, Eisenberger A et al. Doxorubicin, cardiac risk factors, and cardiac toxicity in elderly patients with diffuse B‐cell non‐Hodgkin's lymphoma. J Clin Oncol 2008;26:3159–3165. [DOI] [PubMed] [Google Scholar]
- 9.Prescribing informations for doxorubicin hydrochloride . Available at http://www.bccancer.bc.ca/drug-database-site/Drug%20Index/Doxorubicin_monograph_1Feb2017.pdf. Accessed November 7, 2015
- 10. van Dalen EC, van der Pal HJ, Caron HN et al. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev 2009;(4):CD005008. [DOI] [PubMed] [Google Scholar]
- 11. Tebbi CK, London WB, Friedman D et al. Dexrazoxane‐associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 2007;25:493–500 [DOI] [PubMed] [Google Scholar]
- 12.FDA statement on Dexrazoxane. Available at http://www.fda.gov/Drugs/DrugSafety/ucm263729.htm. Accessed November 7, 2015.
- 13. Mross K, Niemann B, Massing U et al. Pharmacokinetics of liposomal doxorubicin (TLC‐D99; Myocet) in patients with solid tumors: An open‐label, single‐dose study. Cancer Chemother Pharmacol 2004;54:514–524. [DOI] [PubMed] [Google Scholar]
- 14. van Dalen EC, Michiels EM, Caron HN et al. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database Syst Rev: 2010;(5):CD005006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Luminari S, Montanini A, Caballero D et al. Nonpegylated liposomal doxorubicin (MyocetTM) combination (R‐COMP) chemotherapy in elderly patients with diffuse large B‐cell lymphoma (DLBCL): Results from the phase II EUR018 trial. Ann Oncol 2010;21:1492–1499. [DOI] [PubMed] [Google Scholar]
- 16. Visani G, Ferrara F, Alesiani F et al. R‐COMP 21 for frail elderly patients with aggressive B‐cell non‐Hodgkin lymphoma: A pilot study. Leuk Lymphoma 2008;49:1081–1086. [DOI] [PubMed] [Google Scholar]
- 17.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection frations. N Engl J Med 1992;327:685–691. [DOI] [PubMed] [Google Scholar]
- 18. Cardinale D, Colombo A, Bacchiani G et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 19. Cardinale D, Sandri MT, Colombo A et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high‐dose chemotherapy. Circulation 2004;109:2749–2754. [DOI] [PubMed] [Google Scholar]
- 20. Cheson BD, Pfistner B, Juweid ME et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 21. Vitolo U, Chiappella A, Franceschetti S et al. Lenalidomide plus R‐CHOP21 in elderly patients with untreated diffuse large B‐cell lymphoma: Results of the REAL07 open‐label, multicentre, phase 2 trial. Lancet Oncol 2014;15:730–737. [DOI] [PubMed] [Google Scholar]
- 22. Aleman BM, van den Belt‐Dusebout AW, De Bruin ML et al. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood 2007;109:1878–1886. [DOI] [PubMed] [Google Scholar]
- 23. Cardinale D, Sandri MT, Martinoni A et al. Left ventricular dysfunction predicted by early troponin I release after high‐dose chemotherapy. J Am Coll Cardiol 2000;36:517–522. [DOI] [PubMed] [Google Scholar]
- 24. Sandri MT, Cardinale D, Zorzino L et al. Minor increases in plasma troponin I predict decreased left ventricular ejection fraction after high‐dose chemotherapy. Clin Chem 2003;49:248–252. [DOI] [PubMed] [Google Scholar]
- 25. Cardinale D, Colombo A, Sandri MT et al. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation 2006;114:2474–2481. [DOI] [PubMed] [Google Scholar]
- 26. Cardinale D, Colombo A, Lamantia G et al. Anthracycline‐induced cardiomyopathy: Clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol 2010;55:213–220. [DOI] [PubMed] [Google Scholar]
- 27. Curigliano G, Cardinale D, Suter T et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol 2012;23 Suppl 7:vii155–166 [DOI] [PubMed] [Google Scholar]
- 28. Thavendiranathan P, Poulin F, Lim KD et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: A systematic review. J Am Coll Cardiol 2014;63:2751–2768. [DOI] [PubMed] [Google Scholar]
- 29.Gazzetta Ufficiale della Repubblica Italiana del 23/05/2011;152(118):114.
- 30. Fridrik MA, Jaeger U, Petzer A et al. Cardiotoxicity with rituximab, cyclophosphamide, nonpegylated liposomal doxorubicin, vincristine and prednisolone compared to rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisolone in frontline treatment of patients with diffuse large B‐cell lymphoma: A randomised phase‐III study from the Austrian Cancer Drug Therapy Working Group [Arbeitsgemeinschaft Medikamentöse Tumortherapie AGMT] (NHL‐ 14). Eur J Cancer 2016;58:112–121. [DOI] [PubMed] [Google Scholar]
- 31. Bosch X, Rovira M, Sitges M et al. Enalapril and carvedilol for preventing chemotherapy‐induced left ventricular systolic dysfunction in patients with malignant hemopathies: The OVERCOME trial (preventiOn of left Ventricular dysfunction with Enalapril and caRvedilol in patients submitted to intensive ChemOtherapy for the treatment of Malignant hEmopathies). J Am Coll Cardiol 2013;61:2355–2362. [DOI] [PubMed] [Google Scholar]
- 32. Gómez H, Hidalgo M, Casanova L et al. Risk factors for treatment‐related death in elderly patients with aggressive non‐Hodgkin's lymphoma: Results of a multivariate analysis. J Clin Oncol 1998;16:2065–2069. [DOI] [PubMed] [Google Scholar]
- 33. Pfreundschuh, M. How I treat elderly patients with diffuse large B‐cell lymphoma. Blood 2010;116:5103–5110. [DOI] [PubMed] [Google Scholar]
- 34. Olivieri A, Gini G, Bocci C et al. Tailored therapy in an unselected population of 91 elderly patients with DLBCL prospectively evaluated using a simplified CGA. The Oncologist 2012;17:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lotrionte M, Biondi‐Zoccai G, Abbate A et al. Review and meta‐analysis of incidence and clinical predictors of anthracycline cardiotoxicity. Am J Cardiol 2013;112:1980–1984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.