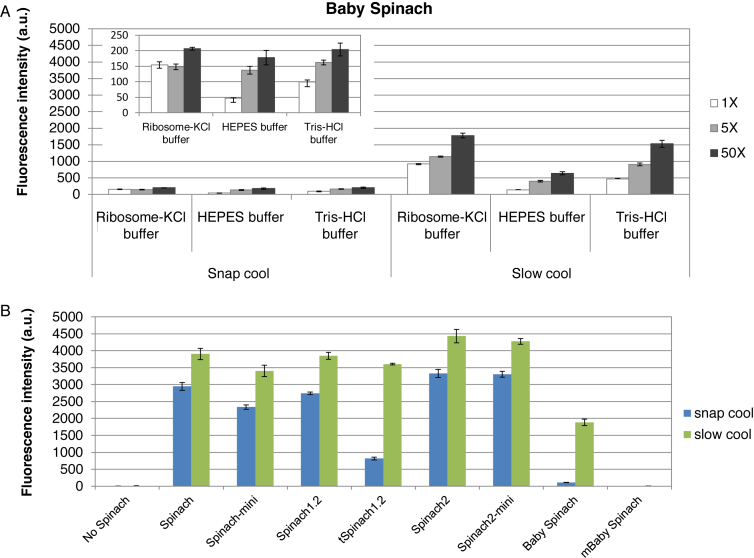
Figure 3.
Effect of folding protocols on fluorescence of Spinach transcripts. (A) Baby Spinach requires slow cooling for efficient folding. For snap cooling, the RNA transcript in water was heated to 90°C, placed on ice for 5 min and then buffer and DFHBI were added. For slow cooling, the buffer and DFHBI were added to the snap-cooled samples; samples were then incubated at 65°C for 5 min and then cooled slowly to 25°C. Fluorescence intensities were determined using 0.2 μM RNA and 0.2 μM (white bars), 1 μM (light grey bars) and 10 μM (dark grey bars) of DFHBI in indicated buffers. Tris-HCl buffer contains 40 mM Tris-HCl (pH 7.5), 125 mM KCl, 5 mM MgCl2 (11). The signals obtained with the snap cool protocol are expanded in the inset. (B) Fluorescence intensities of Spinach sequences in ribosome-KCl buffer in the presence of 50-fold molar excess of DFHBI after folding using the snap cool (blue bars) or slow cool (green bars) protocol. Error bars are s. e. m. for three independent experiments.