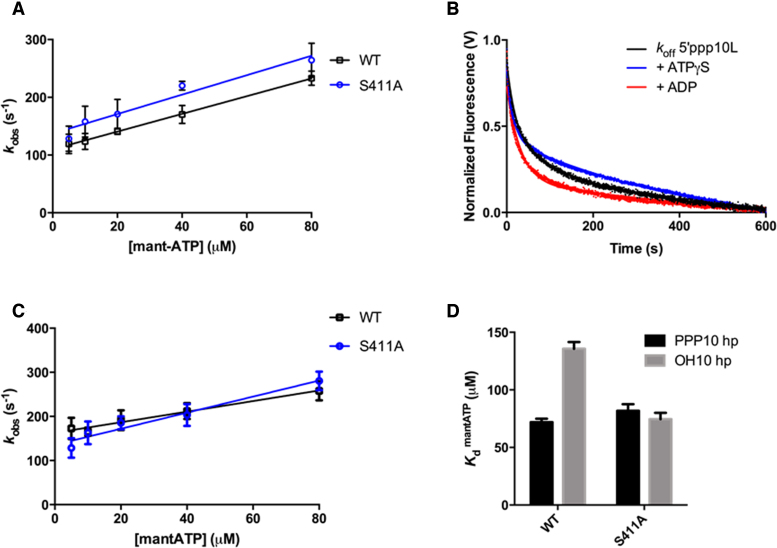
Figure 4.
RNA and ATP binding activities of S411A. (A) Average linear fit of [mant-ATP] versus kobs for WT RIG-I bound to 5΄ppp10L (black) and S411A bound to 5΄ppp10L (red), showing the [mant-ATP] dependence of the observed rate constants. Plotted kobs values are mean ± SD (n = 3). The kon (slope) and koff (intercept) values were derived from three independent trials and averaged. Using these values (koff/kon), a Kd was calculated for each protein/RNA combination. WT RIG-I/5΄ppp10L: koff = 110.0 ± 5, kon = 1.5 ± 0.1, Kd(calc.) = 72 ± 4 μM. S411A/5΄ppp10L: koff = 137.4 ± 10., kon = 1.7 ± 0.2, Kd(calc.) = 82 ± 6 μM. (B) koffRNA traces for S411A. Four traces monitoring displacement of S411A from 5΄ppp10L (black) were averaged and fit to a double exponential equation. Also shown are koff traces in the presence of 3 mM ATPγS (red) and 3 mM ADP (blue). (C) Average linear fit of [mant-ATP] versus kobs for WT RIG-I bound to OH10L (black) and S411A bound to OH10L (red), showing the [mant-ATP] dependence of the observed rate constants. Plotted kobs values are mean ± SD (n = 3). Kd values were calculated as in (A). WT RIG-I/OH10L: koff = 162.8 ± 7, kon = 1.2 ± 0.2, Kd(calc.) = 136 ± 6 μM. S411A/OH10L: koff = 135.7 ± 10, kon = 1.8 ± 0.2, Kd(calc.) = 75 ± 5 μM. (D) Bar plot of extrapolated dissociation constants for MANT-ATP binding by wild type and S411A RIG-I proteins bound to 5΄ppp10L and OH10L.