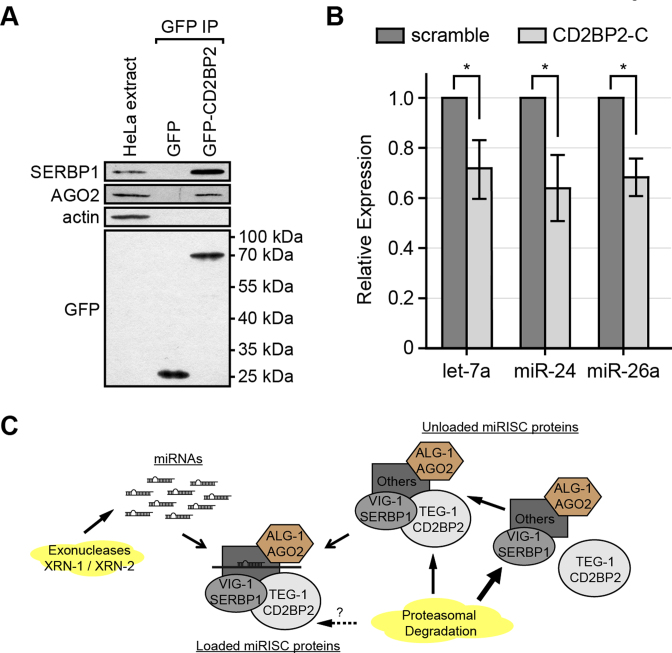
Figure 6.
Association of TEG-1 with miRISC proteins is conserved in human tissue culture cells. (A) SERBP1/PAI-RBP1 (VIG-1 ortholog) and AGO2 (ALG-1 ortholog) are co-IP'd with CD2BP2 (TEG-1 ortholog). GFP or GFP-CD2BP2 was transiently expressed in HeLa cells and proteins were co-IP'd using anti-GFP antibodies. SERBP1/PAI-RBP1 and AGO2 are detected when IP'd with anti-GFP antibodies followed by immunoblotting, while the negative control, actin, was not detected. (B) Mature let-7a, miR-24, and miR-26a miRNAs are significantly reduced in HeLa cells treated with CD2BP2 siRNA in comparison with a scrambled siRNA treatment by quantitative real time PCR using TaqMan probes. A two-tailed Student's t-test was used to determine the statistical significance: *P < 0.0003. Error bar = standard deviation. (C) Proposed model. Free miRNAs are degraded by exonucleases (XRN-1/XRN-2), while miRISC proteins are subjected to proteasomal degradation. Our model suggests that TEG-1 CD2BP2 interacts with miRISC. TEG-1 CD2BP2 helps stabilize miRISC protein components. In the absence of TEG-1 CD2BP2, miRISC protein components are more susceptible to proteasomal degradation (thick arrow), which in turn results in decreased stability of miRNAs.