Figure 2. ZIKV mRNA LNP vaccine protects C57BL/6 mice.
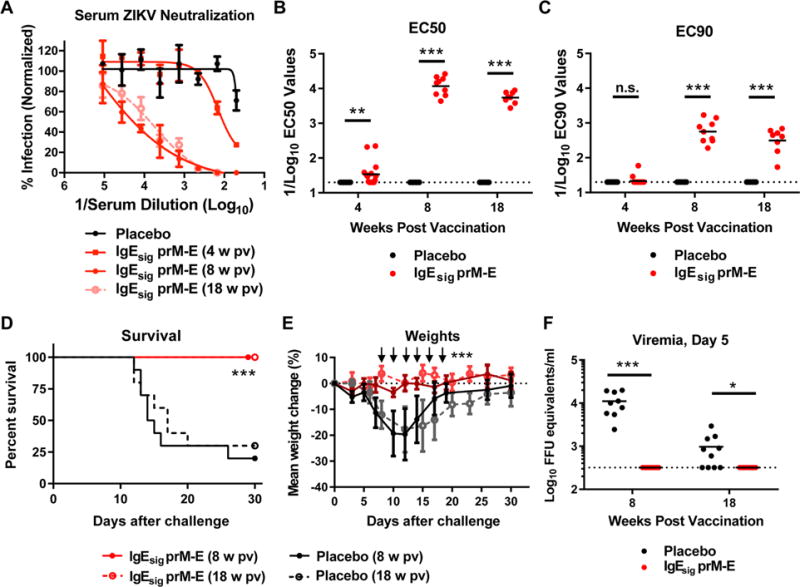
WT C57BL/6 mice (n = 10, pooled from two independent experiments) were immunized with 10 μg of placebo or IgEsig prM-E mRNA LNPs and boosted with an equivalent dose four weeks later. A. Serum was collected at 4, 8, and 18 weeks post initial vaccination and analyzed for ZIKV neutralization activity by FRNT assay. Representative neutralization curves are shown for each group. Error bars denote the standard deviation (SD) of triplicate technical replicates. B–C. EC50 (B) and EC90 (C) values were calculated for individual animals in each group. The dashed lines indicate the limit of detection of the assay. Data were analyzed using the Mann-Whitney test and compared to the placebo LNP vaccine at each time point (**, P < 0.01; ***, P < 0.001; n.s. indicates not significant). D–F. At week 8 or 18, vaccinated C57BL/6 mice were administered 2 mg of anti-Ifnar1 blocking antibody and one day later challenged with 105 FFU of mouse adapted ZIKV Dakar 41519. Animals were monitored for survival (D) and weight loss (E). At day 5 after viral challenge, serum was analyzed for levels of ZIKV RNA (F). The dashed line indicates the limit of detection of the assay. Survival data was analyzed by the log rank test (***, P < 0.001). Weight loss was analyzed by two-way ANOVA (***, P < 0.001) for surviving animals; arrows indicate days having statistically significant differences from placebo vaccine. Viremia data was analyzed by a Mann-Whitney test (*, P < 0.05; ***, P < 0.001). See also related figure S3.
