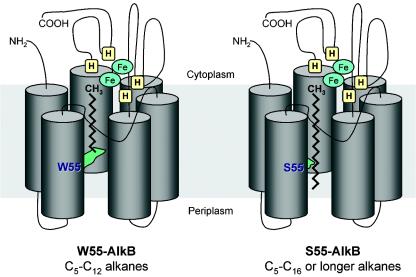
FIG. 1.
Model for the AlkB alkane hydroxylase. The enzyme is proposed to have six transmembrane helices arranged in a hexagonal distribution that would define a long, hydrophobic pocket into which the linear alkane molecule can slip. The four histidine clusters (H) believed to bind the two iron atoms (Fe) would lie on the cytoplasmic side. In P. putida GPo1 AlkB, residue W55 lies in transmembrane helix 2 and extends its bulky arm towards the hydrophobic pocket (left). This hampers the proper insertion of alkanes longer than C13. The replacement of W55 by a serine residue (S55) (right), which has a shorter arm, allows longer alkanes to enter the pocket without impeding the proper alignment of the terminal methyl group relative to the histidine clusters. Sizes are not to scale. The soluble components of the alkane hydroxylase complex, rubredoxin and rubredoxin reductase, are not shown. The model is modified from reference 20 with permission.