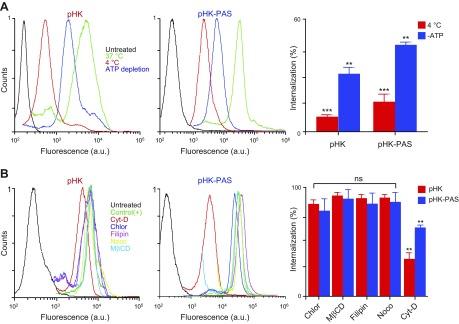
Figure 3.
Determination of cellular uptake mechanisms of pHK and pHK-PAS. A) Effects of low temperature and energy depletion on peptide internalization. HeLa cells were preincubated for 1 h at 4°C in serum-free DMEM or pretreated for 1 h at 37°C with 10 mM sodium azide and 6 mM 2-deoxy-d-glucose in serum- and glucose-free DMEM to deplete cellular ATP. pHKA488 (25 µM; left) or pHK-PASA488 (25 µM; middle) was then added, and cells were maintained for 2 h at 4°C or in the presence of sodium azide/2-deoxy-d-glucose at 37°C. B) Effects of endocytosis inhibitors on peptide internalization. HeLa cells were treated for 30 min at 37°C in serum-free DMEM with the following: 10 µM chlorpromazine (Chlor; clathrin-dependent endocytosis), 5 mM methyl-β-cyclodextrin (MβCD; lipid raft–mediated endocytosis), 4 µM filipin (Filip; caveolae-dependent endocytosis), 10 µM nocodazole (Nocod; microtubule polymerization), or 10 µM cytochalasin D (Cyto D; macropinocytosis). Cells were then treated with 25 µM pHKA488 (left) or pHK-PASA488 (middle) and maintained for 2 h at 37°C in the presence of inhibitors and peptides. Thereafter, cells were washed 3 times with ice-cold PBS, trypsinized, centrifuged, and resuspended in ice-cold PBS with 10% FBS, and fluorescence was measured by FACS. Cells that were treated with peptide without inhibitors at 37°C were used as control, and cells that were treated with vehicle alone served as background. Uptake efficiencies (right) were determined from the ratio of fluorescence of cells treated with peptide under different inhibition conditions to control cells. ns, nonsignificant (P > 0.05). **P < 0.001, ***P < 0.0001 compared with controls at 37°C.