Abstract
Longitudinal opioid prescription use is unknown among HIV-infected patients. Group-based trajectory modeling followed by multinomial logistic regression was used to identify distinct trajectories and their association with baseline characteristics among 1239 HIV-infected UNC CFAR HIV Clinical Cohort participants, 2000–2014. Three trajectories were identified: 1) 72% never/sporadic opioid use (referent group), 2) 11% episodic use (associated with female sex, depression, drug-related diagnoses, antiretroviral therapy use and undetectable HIV RNA), and 3) 16% chronic use (associated with older age, female sex and mental health diagnoses). Overall, opioid prescription decreased substantially with longer time in HIV care among both episodic and chronic users.
Keywords: HIV, Opioids, Group-based trajectory analysis, Cohort
INTRODUCTION
Both therapeutic use and misuse of opioids have escalated over the last few decades in the United States.1 The number of persons receiving an opioid prescription more than doubled in the Veterans Aging Cohort Study-Virtual Cohort between 1999–2010, among both HIV-infected and uninfected participants.2 HIV-infected patients may be prescribed opioids twice as often as HIV-uninfected persons.3 While guidelines for opioid prescription have been issued by the Centers for Disease Control and Prevention,4 guideline-concordant care is inconsistent among HIV-infected patients.5 Although the increased risks of misuse,6 mortality,7,8 and other adverse health outcomes9–11 associated with opioid use have been established, longitudinal opioid use has not been well defined and little is known about changes in HIV-infected individuals’ use over time. The objectives of this exploratory study were to identify patterns of longitudinal opioid prescription use and to assess their association with patient characteristics.
METHODS
The University of North Carolina Center for AIDS Research HIV Clinical Cohort (UCHCC) is an ongoing prospective cohort of all patients receiving primary HIV care at the University of North Carolina Hospitals. The UCHCC study and procedures have been previously described and the cohort is representative of HIV-infected patients in care in North Carolina.12 The analytic sample consisted of all UCHCC participants in care (i.e. at least one clinic visit or CD4 cell count/HIV viral load test per year) for a minimum of five years between 2000 and 2014. Baseline was defined as the first year in care at UNC hospitals in or after 2000.
Each year, participants were categorized based on their opioid prescription receipt. Opioids considered were codeine, hydrocodone, hydromorphone, morphine, oxycodone, oxymorphone, propoxyphene, tramadol and fentanyl. Only medications with pain management indication were included (e.g. cough suppressants containing codeine were excluded). Methadone was excluded (n=13) because the indication was unknown (e.g. substitution therapy or chronic pain). Data on prescription medication use during each calendar year as well as sociodemographic and clinical characteristics were obtained from electronic medical records and supplemented by standardized medical record reviews. No information on the specific date, dose and duration of opioid prescription was available.
Group-based trajectory models,13 a longitudinal extension of latent-class analysis, were used to map the course of opioid use over time and identify clusters of individuals following similar trajectories. Models with 1–6 groups were fitted successively and compared to select the optimal number of trajectories. Model selection was based on model fit, adequacy and interpretability. Fit was assessed with the Akaike information criterion (AIC) and the Bayesian information criterion (BIC). Adequacy was assessed with correspondence between the estimated probability of group membership and the proportion assigned to the group, and an average posterior probability of group membership ≥0.70.14 Group membership was assigned based on each patient’s highest posterior probability of belonging to a specific group.
Predicted probabilities of opioid use within each trajectory group were plotted against the number of years in care. Group membership was used as the outcome in a multivariate multinomial logistic regression to assess its relationship with sociodemographic and clinical characteristics at baseline (i.e. first year of HIV care at UNC in or after 2000). Selected characteristics included in a fully adjusted model were diagnoses of chronic pain, alcohol use- and any drug use-related diagnoses, depression, mental illness (excluding depression), sex, race, age, antiretroviral therapy (ART) use, HIV RNA suppression (HIV RNA <50 copies/ml throughout the year) and CD4 cell count (lowest value for the year).
RESULTS
Overall 1,239 HIV-infected patients had at least five years of HIV care follow-up between 2000 and 2014. The median calendar year of entry to the cohort was 2005 (Interquartile range [IQR], 2002–2008), and the median years contributed to follow-up was 9 (IQR, 7–12), with a total of 11,764 person-years of follow-up. Patients were predominantly black (60%), male (70%), with a median age of 39 years at baseline (Table 1). At baseline, the median CD4 cell count was 306 cells/mm3, 48% used ART, and 14% were virologically suppressed.
Table 1.
Baseline patient characteristics and associations with longitudinal opioid use using never/sporadic use (n=898, 72%) as the referent outcome in a multivariate multinomial logistic regression model
| Baseline Characteristics N (%) or median (IQR) | Opioid Use Group |
||
|---|---|---|---|
| Episodic use aOR (95% CI) |
Chronic use aOR (95% CI) |
||
| N (%) | 1239 (100) | 141 (11) | 200 (16) |
|
| |||
| Female sex | 372 (30) | 1.82 (1.17, 2.83)* | 2.86 (1.71, 4.79)* |
|
| |||
| Race | |||
| White | 366 (30) | Ref. | Ref. |
| Black | 739 (60) | 1.45 (0.89, 2.34) | 0.90 (0.52, 1.54) |
| Other | 134 (11) | 0.88 (0.40, 1.94) | 0.49 (0.16, 1.48) |
|
| |||
| Age (years) | 39 (24–46) | 1.19 (0.98, 1.46) | 1.37 (1.08, 1.75)* |
|
| |||
| CD4 cell count (cells/mm3) | 306 (144–499) | 0.99 (0.92, 1.08) | 0.93 (0.84, 1.03) |
|
| |||
| ART use | 589 (48) | 1.74 (1.12, 2.68)* | 1.39 (0.80, 2.42) |
|
| |||
| Suppressed HIV RNAa | 175 (14) | 3.28 (1.48, 7.26)* | 1.24 (0.58, 2.69) |
|
| |||
| Chronic painb | 25 (2) | 1.44 (0.32, 6.48) | 2.84 (0.73, 11.00) |
|
| |||
| Alcohol-related diagnosisb | 69 (6) | 1.15 (0.43, 3.09) | 1.98 (0.72, 5.50) |
|
| |||
| Drug-related diagnosisb | 157 (13) | 2.18 (1.11, 4.28)* | 1.74 (0.80, 3.78) |
|
| |||
| Depressionb | 150 (12) | 2.89 (1.42, 5.87)* | 1.55 (0.69, 3.50) |
|
| |||
| Mental illnessb | 85 (7) | 0.88 (0.23, 3.38) | 5.60 (2.55, 12.27)* |
Defined as HIV viral load <50 copies/ml
Defined as the presence of a physician diagnosis of chronic pain, alcohol use disorder, drug use disorder, depression or another mental illness (excluding depression) and recorded in patient’s medical records
p-value <0.05
The three-groups model provided the best fit to the data. The first group consisted of patients with never/sporadic opioid use, with median 7% of follow-up years with opioid use (IQR, 0–17%). The second group consisted of patients with episodic use who received at least one opioid prescription in a minimum of 2 consecutive years, with median 30% of follow-up years with opioid use (IQR, 25–38%). The third group consisted of patients with chronic use who received opioid prescriptions for at least half the years in care, with median 63% of follow-up years with opioid use (IQR, 54–80%). This model provided the closest correspondence between the estimated probability of group membership and the proportion assigned to each group, with values of 0.64/0.72, 0.19/0.11 and 0.16/0.16 for the never/sporadic, episodic and chronic use groups respectively. The mean posterior probability was good in each group at 0.85, 0.75 and 0.88, for the never/sporadic, episodic and chronic use groups respectively. Only a marginal improvement in model fit (AIC and BIC) was observed in models with more than three groups. Finally, the three-group model decreased heterogeneity within groups and increased heterogeneity between groups.
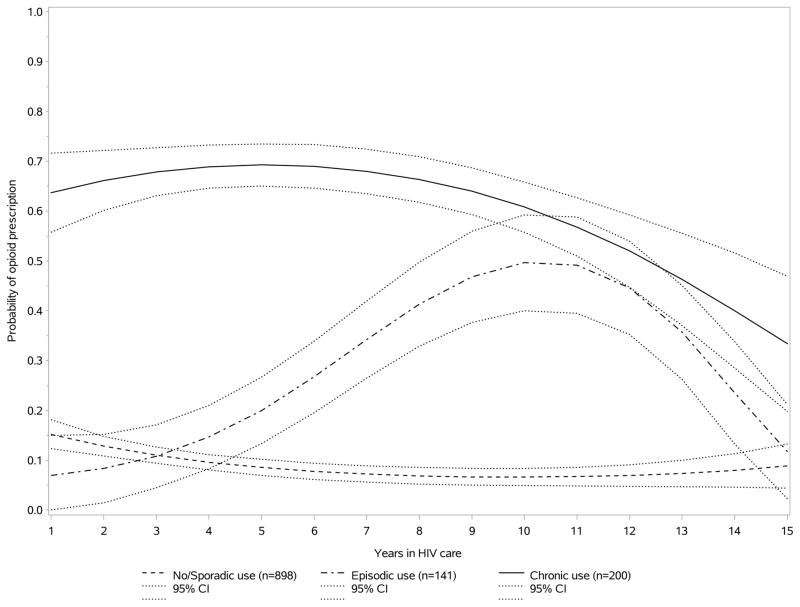
With this model, 898 patients (72%) were assigned to the never/sporadic, 141 (11%) to the episodic and 200 (16%) to the chronic use group. The estimated predicted probability of opioid use decreased at first then stabilized over time among never/sporadic users, increased and then decreased over time among episodic users, whereas the probability of use was stable and then decreased with time among chronic opioid users (Figure 1). Among the never/sporadic users, the predicted probabilities of opioid use at 1, 5, 10 and 15 years of care respectively were 16% (95% Confidence Interval [CI], 13–18%), 8% (7–10%), 8% (6–10%) and 6% (2–11%). Among the episodic users, these probabilities were 7% (0–15%), 22% (14–30%), 51% (41–62%) and 14% (2–25%), and among the chronic users, 64% (55–73%), 69% (64–74%), 60% (54–66%) and 35% (15–55%) (estimated flexibly with statistically significant quadratic or cubic functions).
Figure 1.
Probability of longitudinal opioid use among HIV-infected patients with never/sporadic, episodic and chronic opioid use, estimated using a group-based trajectory model.
A number of baseline characteristics were associated with longitudinal opioid use in a multivariate multinomial logistic regression model (Table 1). Compared to never/sporadic users, episodic users were more likely to be female (Odds Ratio [OR], 1.82, 95% CI, 1.17–2.83), depressed (2.89, 1.42–5.87), have a drug-related diagnosis (2.18, 1.11–4.28), use ART (1.74, 1.12–2.68) and have suppressed HIV RNA (3.28, 1.48–7.26). Chronic users were more likely to be female (2.86, 1.71–4.79), be older (1.37 per 10 years, 1.08–1.75) and have a mental illness (5.60, 2.55–12.27).
DISCUSSIONS
To our knowledge, this is the first investigation of longitudinal opioid use among HIV-infected patients receiving HIV care. We identified three distinct patterns of opioid use over time, including the most common of never/sporadic use, followed by chronic and episodic use. The probability of using opioids changed over time among both episodic and chronic users, with eventual decreases in probability of opioid use with longer time in care in both groups. Decreasing opioid use may be in part related to overall decreases in opioid prescription among this clinical population in more recent calendar years or to regression to the mean. This trend could also be explained by a shift towards earlier ART initiation in more recent years, potentially resulting in fewer patients with comorbid conditions necessitating pain management or better control of underlying comorbidities resulting from longer engagement in care, as well as, greater emphasis placed on the implementation of existing opioid agreements in the clinic starting in 2012.
The only factor associated with both episodic and chronic use, compared to never/sporadic use was female sex. Additionally, ART use, HIV RNA suppression, and a drug-related or depression diagnosis at baseline was associated with episodic opioid use. Older age and a mental health diagnosis other than depression at baseline was associated with chronic opioid use.
Most participants rarely received opioid prescription, which were therefore likely used for short-term treatment of acute pain (e.g. following an illness, injury or surgery). Although estimates of chronic pain prevalence in HIV-infected populations range from 53–90%,15,16 only 16% received chronic opioid treatment in this cohort. We were unable to assess receipt of alternate pain management. Chronic use group membership was associated with mental illness other than depression, female sex and older age. Female sex3,17 and mental illnesses such as anxiety and post-traumatic stress disorder 2,17,18 have previously been associated with opioid use among HIV-infected individuals, although individuals with schizophrenia are less likely to receive opioids.2 Older age has been associated with both an increase2,3,18 and a decrease in the risk of opioid use.17 Drug-related diagnoses were associated with the episodic use group in this study. Both substance use disorders and history of drug use have been associated with long-term, daily, or high dose opioid use in some studies of HIV-infected persons.2,3,19 However, other studies have found either no association with any or chronic opioid prescription,17,18 or a protective association for any opioid receipt among HIV-infected persons.2
Only 9% of the study population were episodic users. We were unable to assess whether these patients were receiving opioids for ongoing comorbidities, or whether they had ongoing chronic pain that was alternately being managed with different strategies. It is also possible that some of these patients used opioids prescribed by other providers without the knowledge of their primary HIV care provider.
Though pain-related diagnoses have previously been associated with opioid use,2,17,18 a baseline chronic pain diagnosis was not associated with specific trajectories of opioid use in this study. However, only 2% of patients had a chronic pain diagnosis at baseline, which could explain the elevated point estimates, but wide confidence intervals observed. This low prevalence is likely an underestimation in this study, given prior estimates,15,16 and could result in part from the use of chart reviews rather than patient self-report of chronic pain. Additionally, we only included a chronic pain diagnosis at beginning of follow-up, rather than including all chronic pain diagnoses occurring during follow-up. Although studies have shown that white HIV-infected persons are more likely to receive long-term and high-dose opioid prescriptions in very large cohorts of HIV-infected persons,2,3,17 no such association was identified for opioid prescription trajectories in this study or with daily or chronic opioid prescription in other smaller HIV cohorts.18,19 Reduced power is therefore a possible explanation for this discrepancy.
The UCHCC is a large cohort representative of the HIV-infected population in North Carolina, which relies on electronic records and standardized health record reviews, resulting in rich data on HIV and other co-morbidities. However, opioid prescription practices may vary across the United States and these results may not be generalizable to all regions. Limited sociodemographic and behavioral information was available. Alcohol and drug use-related diagnoses in the clinical record were included, although these diagnoses likely underestimate true prevalence. Limited information on opioid prescription was available; only yearly opioid use could be assessed, without information on the dose, reason for prescription, or prescription receipt from another source. In addition, this study focused on prescription receipt and no inference can be made on actual use or misuse of these prescriptions.
Censoring of person-time could have affected grouping in group-based trajectory models. Follow-up time was censored administratively on January 1, 2015 and when a patient did not have a visit for over one year (loss to follow-up). The absence of information on opioid use after censoring can result in grouping misclassification. To minimize such misclassification and to ensure that sufficient data was available to inform the trajectory of opioid use over time, the analytic sample was limited to patients with a minimum of five years in care.
This first study of patterns of opioid use over time in a representative cohort of HIV-infected persons engaged in clinical care identified three distinct trajectories: never/sporadic, episodic and chronic use. A notable group of patients used opioids chronically and episodically, although the overall probabilities of use decreased with longer time in HIV care. Members of the chronic use group were more likely to be older, female and to be diagnosed with mental illness. These findings highlight the need to further identify pharmacologic and non-pharmacologic treatments for chronic pain among HIV-infected patients, targeted towards vulnerable groups such as women and patients with mental illness.
Acknowledgments
Source of Funding: This study was funded by the University of North Carolina at Chapel Hill Center for AIDS Research (CFAR), an NIH funded program P30 AI50410. LB is receiving a postdoctoral scholarship from the Canadian Institutes for Health Research. JJE has received research support to UNC-CH as an investigator from ViiV Healthcare, Janssen, Gilead Sciences, Bristol-Myers Squibb. Consulting was paid to JJE by ViiV Healthcare, Janssen, Gilead Sciences, Bristol-Myers Squibb, Merck.
The authors thank all participants, clinicians, investigators, and staff involved with the UNC CFAR HIV clinical cohort.
Footnotes
Data presented at: 20th International Workshop on HIV Observational Databases, Budapest, Hungary, April 7–9, 2016.
References
- 1.Manchikanti L, Fellows B, Ailinani H, Pampati V. Therapeutic use, abuse, and nonmedical use of opioids: a ten-year perspective. Pain physician. 2010;13(5):401–435. [PubMed] [Google Scholar]
- 2.Becker WC, Gordon K, Jennifer Edelman E, et al. Trends in Any and High-Dose Opioid Analgesic Receipt Among Aging Patients With and Without HIV. AIDS Behav. 2016;20(3):679–686. doi: 10.1007/s10461-015-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silverberg MJ, Ray GT, Saunders K, et al. Prescription long-term opioid use in HIV-infected patients. Clin J Pain. 2012;28(1):39–46. doi: 10.1097/AJP.0b013e3182201a0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. CDC Guideline for Prescribing Opioids for Chronic Pain — United States, 2016. Morbidity and Mortality Weekly Report. 2016;65(1) doi: 10.15585/mmwr.rr6501e1. [DOI] [PubMed] [Google Scholar]
- 5.Gaither JR, Goulet JL, Becker WC, et al. Guideline-concordant management of opioid therapy among human immunodeficiency virus (HIV)-infected and uninfected veterans. The journal of pain: official journal of the American Pain Society. 2014;15(11):1130–1140. doi: 10.1016/j.jpain.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557–564. doi: 10.1097/AJP.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA: the journal of the American Medical Association. 2011;305(13):1315–1321. doi: 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 8.Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171(7):686–691. doi: 10.1001/archinternmed.2011.117. [DOI] [PubMed] [Google Scholar]
- 9.Dublin S, Walker RL, Jackson ML, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc. 2011;59(10):1899–1907. doi: 10.1111/j.1532-5415.2011.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders KW, Dunn KM, Merrill JO, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. Journal of general internal medicine. 2010;25(4):310–315. doi: 10.1007/s11606-009-1218-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon DH, Rassen JA, Glynn RJ, Lee J, Levin R, Schneeweiss S. The comparative safety of analgesics in older adults with arthritis. Arch Intern Med. 2010;170(22):1968–1976. doi: 10.1001/archinternmed.2010.391. [DOI] [PubMed] [Google Scholar]
- 12.Napravnik S, Eron JJ, Jr, McKaig RG, Heine AD, Menezes P, Quinlivan E. Factors associated with fewer visits for HIV primary care at a tertiary care center in the Southeastern U.S. AIDS Care. 2006;18(6):45–50. doi: 10.1080/09540120600838928. [DOI] [PubMed] [Google Scholar]
- 13.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–393. [Google Scholar]
- 14.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 15.Uebelacker LA, Weisberg RB, Herman DS, Bailey GL, Pinkston-Camp MM, Stein MD. Chronic Pain in HIV-Infected Patients: Relationship to Depression, Substance Use, and Mental Health and Pain Treatment. Pain Med. 2015;16(10):1870–1881. doi: 10.1111/pme.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. The journal of pain: official journal of the American Pain Society. 2011;12(9):1004–1016. doi: 10.1016/j.jpain.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edelman EJ, Gordon K, Becker WC, et al. Receipt of opioid analgesics by HIV-infected and uninfected patients. Journal of general internal medicine. 2013;28(1):82–90. doi: 10.1007/s11606-012-2189-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Merlin JS, Tamhane A, Starrels JL, Kertesz S, Saag M, Cropsey K. Factors Associated with Prescription of Opioids and Co-prescription of Sedating Medications in Individuals with HIV. AIDS Behav. 2015 doi: 10.1007/s10461-015-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koeppe J, Armon C, Lyda K, Nielsen C, Johnson S. Ongoing pain despite aggressive opioid pain management among persons with HIV. Clin J Pain. 2010;26(3):190–198. doi: 10.1097/AJP.0b013e3181b91624. [DOI] [PubMed] [Google Scholar]