Table 2.
Electrophile-mediated cyclization of N-propargylthioureas 55.
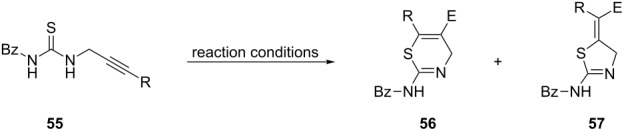 | |||||
| Entry | R | Reaction conditions | E+ |
56 Yield (%) |
57 Yield (%) |
| 1 | H | I2 (2 equiv), DCM, 0 °C | I+ | – | 20 |
| 2 | Ph | I2 (2 equiv), DCM, 0 °C | I+ | 18 | 25 |
| 3 | 4-OMe-Ph | I2 (2 equiv), DCM, 0 °C | I+ | 59 | traces |
| 4 | Ph | NBS (1.1 equiv), DCM, rt | Br+ | – | 67 |
| 5 | 4-OMe-Ph | NBS (1.1 equiv), DCM, rt | Br+ | – | 68 |
| 6 | 4-Cl-Ph | NBS (1.1 equiv), DCM, rt | Br+ | – | 44 |
| 7 | H | PhSeCl (1 equiv), DCM, 0 °C | PhSe+ | – | 45 |
| 8 | Ph | PhSeCl (1 equiv), DCM, 0 °C | PhSe+ | 56 | traces |
| 9 | 4-OMe-Ph | PhSeCl (1 equiv), DCM, 0 °C | PhSe+ | 73 | – |
| 10 | 4-Cl-Ph | PhSeCl (1 equiv), DCM, 0 °C | PhSe+ | 51 | – |
| 11 | H | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C | 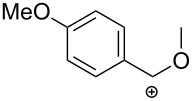 |
– | – |
| 12 | Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C | 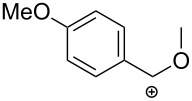 |
81 | – |
| 13 | Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C | 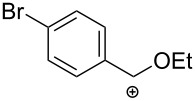 |
60 | – |
| 14 | Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C | 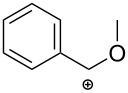 |
57 | – |
| 15 | 4-OMe-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C | 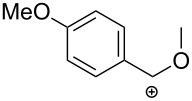 |
76 | – |
| 16 | 4-OMe-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C | 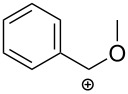 |
59 | – |
| 17 | 4-OMe-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C | 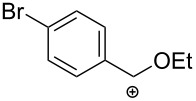 |
58 | – |
| 18 | 4-Cl-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C | 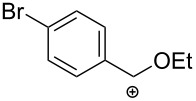 |
57 | – |
| 19 | 4-Cl-Ph | ArCH(OR1)2 (1.5 equiv), TMSOTf (1 equiv), DCM, −10 °C | 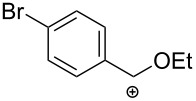 |
65 | – |
