Abstract
Background:
Experimental models using short-duration noxious stimuli have led to the concept of preemptive analgesia. Ketorolac, a nonsteroidal anti-inflammatory drug, has been shown to have a postoperative narcotic-sparing effect when given preoperatively and alternatively to not have this effect. This study was undertaken to determine whether a single intravenous (IV) dose of ketorolac would result in decreased postoperative pain and narcotic requirements.
Methods:
In a double-blind, randomized controlled trial, 48 women undergoing abdominal hysterectomy were studied. Patients in the ketorolac group received 30 mg of IV ketorolac 30 min before surgical incision, while the control group received normal saline. The postoperative analgesia was performed with a continuous infusion of tramadol at 12 mg/h with the possibility of a 10 mg bolus for every 10 min. Pain was assessed using the visual analog scale (VAS), tramadol consumption, and hemodynamic parameters at 0, 1, 2, 4, 8, 12, 16, and 24 h postoperatively. We quantified times to rescue analgesic (morphine), adverse effects, and patient satisfaction.
Results:
There were neither significant differences in VAS scores between groups (P > 0.05) nor in the cumulative or incremental consumption of tramadol at any time point (P > 0.05). The time to first requested rescue analgesia was 66.25 ± 38.61 min in the ketorolac group and 65 ± 28.86 min in the control group (P = 0.765). There were no significant differences in systolic blood pressure (BP) between both groups, except at 2 h (P = 0.02) and 4 h (P = 0.045). There were no significant differences in diastolic BP between both groups, except at 4 h (P = 0.013). The respiratory rate showed no differences between groups, except at 8 h (P = 0.017), 16 h (P = 0.011), and 24 h (P = 0.049). These differences were not clinically significant. There were no statistically significant differences between groups in heart rate (P > 0.05).
Conclusions:
Preoperative ketorolac neither showed a preemptive analgesic effect nor was it effective as an adjuvant for decreasing opioid requirements or postoperative pain in patients receiving IV analgesia with tramadol after abdominal hysterectomy.
Keywords: Abdominal hysterectomy, ketorolac, nonsteroidal anti-inflammatory drugs, postoperative pain, preemptive analgesia
Introduction
Damage to tissues has been shown to provoke a magnified reaction to noxious stimuli, peripherally by diminishing the threshold of nociceptive afferent nerve terminals and centrally by augmenting the excitability of second-order sensory neurons in the spinal cord,[1] later resulting in an amplification and extension of postoperative pain after surgery.[2] Hence, much research has focused on procedures to avoid these central neuroplastic changes through the usage of preemptive analgesia.[3,4] Experimental models have conducted the idea of “preemptive analgesia”.[5] The decrement of afferent nociceptive inputs to the spinal cord using analgesic techniques started before the initial painful stimulus prevents or attenuates the formation of spinal hyperexcitability and prevents the transformed processing of afferent input, leading to less postoperative pain.[6,7] Whether such experimental models apply to the noxious circumstances occurring during surgery is controversial.[8,9,10,11]
Although preemptive analgesia with different agents has been successful in experimental animal models, conclusions from human studies remain controversial.[12] A diversity of agents have been analyzed for their conceivable preemptive analgesic effects:[1] local anesthetics,[13] nonsteroidal anti-inflammatory drugs (NSAIDs),[14,15,16] paracetamol,[17] opioids,[18,19] magnesium,[20] cytokine synthesis inhibitors,[21,22] ketamine,[23] and tricyclic antidepressants.[24]
Scientific research enabling an understanding of the molecular mechanisms of nociception has disclosed considerable functions of cytokines and prostaglandins (PG).[25] Hyperexcitability also appears peripherally in nerve endings at the location of surgical tissue damage and it is mediated in part by PGs.[26,27] Evidence is accumulating that products of the cyclooxygenase (COX-1) pathway may be engaged in the elaboration of central sensitization.[28,29] Drugs that block the formation of PGs such as NSAIDs might, therefore, be assumed to avoid or minimize the formation of this peripheral and central hyperexcitability.[5] Their central analgesic actions are effected by averting spinal PG synthesis and attenuating liberation of neurotransmitters from the primary afferent terminals and spinal interneurons.[30]
Sporadic studies have established some considerable preemptive benefits of NSAIDs.[31] As a result, the objective of this study was to ascertain the impact of a NSAID, ketorolac, on pain severity and analgesic requirement in the early postoperative period.
Ketorolac is a nonselective NSAID that blocks COX-1 and COX-2 enzymes and as a result, blocks the formation of PGs attenuating the sensitization procedures. The antinociceptive and anti-inflammatory actions of NSAIDs may be associated to the suppression of nitric oxide synthase activation,[32,33] decreased generation of proinflammatory cytokines,[34] and lipoxine activation.[35] Consequently, this multidirectional activity indicates that there may be the probability of adjusting the nociception process by the employment of these drugs perioperatively.[25]
To our knowledge, no prior controlled study has determined the effectiveness of preoperative intravenous (IV) ketorolac compared to placebo in patients who underwent abdominal hysterectomies. Thus, this clinical trial was conceived to explore the postoperative analgesic efficiency and opioid-sparing action of a single dose of IV ketorolac in contrast with placebo administered preoperatively.
Materials and Methods
Following authorization from the Institutional Ethics Committee of the C. Hospitalario Arquitecto Marcide-Profesor Novoa Santos (Protocol Code: KP 359352) and according to Helsinki, Tokyo, and Venetia statements, 48 women undergoing general anesthesia for abdominal hysterectomies at the C. Hospitalario Arquitecto Marcide-Profesor Novoa Santos were studied. This was a randomized controlled clinical trial. The trial was registered prospectively (ClinicalTrials.gov Identifier: NCT02642718 [December 29, 2015]).
The study candidates were identified from the surgery schedule and approached for consent 1–7 days prior to surgery. All patients gave written, informed consent after receiving an explanation of the aims, procedures, and potential risks of the study. Procedures included total abdominal hysterectomy, with or without salpingo-oophorectomy.
Inclusion criteria included age between 18 and 70 years, normal height and weight, American Society of Anesthesiologists (ASA) Class I, II, and III, elective surgery, surgery time between 30 and 150 min, understanding of the visual analog scale (VAS), no allergies or intolerance to NSAIDs or anesthetics, and no psychiatric illness. Exclusion criteria included renal deterioration, a history of peptic ulceration, asthma, coagulopathy, cognitive impairment, inability to use the patient-controlled analgesia (PCA) device, a history of chronic pain syndromes, a history of chronic use of analgesics, sedatives, opioids, or steroids, liver or hematologic disease, a history of drug or alcohol abuse, and therapy with NSAIDs, anticoagulants, or lithium.
Patients were educated preoperatively about the VAS for pain estimation and on the use of the PCA device. The VAS constitutes a scale with end points of 0 (no pain) and 10 (worst imaginable pain). The patients were randomly allocated to one of the two treatment groups. Randomization was based on computer-generated, random block codes kept in successively numbered envelopes and arranged in a double-blind manner. Pharmacy-prepared 50 mL solutions containing either ketorolac (30 mg) or placebo were given to anesthesiologists. Neither anesthesiologists nor patients were acquainted of the treatment groups. An investigator, unaware of the treatment groups and not participating in patients’ intraoperative care, performed the postoperative assessments.
Patients were premedicated with metoclopramide 10 mg v.o. and ranitidine 300 mg v.o. the night before and at 7.00 am on the day of surgery. Diazepam 5–10 mg v.o. was administered the night before surgery. Two gram of amoxicillin/clavulanic acid was administered IV 1 h before the surgery as antimicrobial prophylaxis. In the operating room, patients in the ketorolac group received 30 mg of ketorolac tromethamine in 0.9% saline IV 30 min from the anesthesiologist before surgical incision. In the control group, 50 ml of 0.9% saline was injected. Besides routine monitoring, the patients were monitored with spectral entropy through an Entropy Module (M-Entropy™; Datex-Ohmeda, Helsinki, Finland) and muscle relaxation (M-NMT module).
After premedication with atropine 0.01 mg/kg, if necessary, general anesthesia was induced with propofol 1–2 mg/kg (or thiopental 6 mg/kg), remifentanil at 0.5 μg/kg/min IV (0.25 μg/kg/min if over 65 years), and muscle relaxation with succinylcholine 1 mg/kg or cisatracurium 0.15 mg/kg. Anesthesia was maintained with nitrous oxide 50% and sevoflurane 0.5%–1% in 50% oxygen, remifentanil in continuous infusion at 0.5–1 μg/kg/min, and neuromuscular blockage with cisatracurium in bolus 0.06 mg/kg on demand. Anesthesia was tailored to keep arterial blood pressure and heart rate within 20% of preinduction levels. About 30 min before the surgical closure, 100 mg of tramadol was administered IV; a continuous infusion of tramadol (PCA) was initiated at 12 mg/h and programed to deliver a bolus of 10 mg of tramadol on demand, with a lockout interval of 10 min. The infusion of remifentanil was stopped at the end of the surgery. Decurarization, if necessary, was accomplished with atropine 0.01 mg/kg and neostigmine 0.03 mg/kg. The use of opioid reversal agents or any other analgesic other than the ones studied was not permitted. In addition, treatments that could interfere with the pain estimation were not allowed. Patients were extubated in the operating room and transferred to the Postanesthesia Care Unit (PACU).
Pain severity was ascertained at time 0 (at arrival at the PACU) and 1, 2, 4, 8, 12, 16, and 24 h postoperatively. Pain was scored using the VAS. If VAS >5, a rescue dose of 0.1 mg/kg morphine was administered IV. The cumulative amounts of tramadol administered through the PCA as a basal constant infusion and incremental amounts of the supplemental bolus required by the patients were recorded at the same time points. Hemodynamic parameters such as systolic blood pressure (BP), diastolic BP, heart rate, and respiratory rate were ascertained at these same time points. The interval time to request of analgesia and the number of times a rescue dose was injected in the first 24 h were recorded. Global patient satisfaction (0–3), concerning pain control, was evaluated 24 h after the operation. All adverse effects and their characteristics were recorded.
Prior to the study, we approximated the sample size required for testing the hypothesis that tramadol consumption and postoperative pain would be less in the ketorolac group than in the control group. A mean difference in VAS scores of 55% (1.65 VAS scores) between groups in the first 24 h postoperatively was defined as clinically important, assuming VAS score of 3 ± 2 in the control group. This criterion was based on the results of a previous pilot study at our institution using the same surgical population and the same outcomes. The needed sample size to show this reduction was estimated as 24 patients per group, giving a statistical power of 0.80 and a type I error protection of 0.05.
We completed a descriptive analysis, presenting the numerical variables as mean ± standard deviation and the categorical variables as integer values and percentages.
Categorical variables were compared between groups with Chi-squared test. Numerical variables were compared between groups, after checking the assumption of normal distribution with the Kolmogorov–Smirnov test, with Student's t-test or Mann–Whitney U-test, accordingly.
Variables in the different time points were compared with Friedman test for related groups. The level of significance was set at P < 0.05. Data were analyzed using IBM SPSS Statistics for Windows, Version 19.0 (Armonk, NY: IBM Corp.).
Results
A total of 48 patients were recruited. All patients completed the study. All patients were discharged and no patients presented any severe postoperative complication.
There were no significant differences between the two groups in demographics such as ASA group (P = 0.290), age (P = 0.532), or weight (P = 0.356) [Table 1]. The two groups did not differ in terms of duration of the surgical procedure (P = 0.791) and intraoperative doses of remifentanil (P = 0.953) [Table 2].
Table 1.
Demographic data.

Table 2.
Intraoperative analgesic data and duration of surgery.

There were no statistically significant differences in VAS scores between the groups. We could see a significant effect of time in pain scores in both groups separately (P < 0.001) [Figure 1]. On arrival at the PACU, pain intensity was higher in the control group, peaking at 1 h but with higher scores for the ketorolac group. We could observe a progressive decrease in pain scores afterward.
Figure 1.
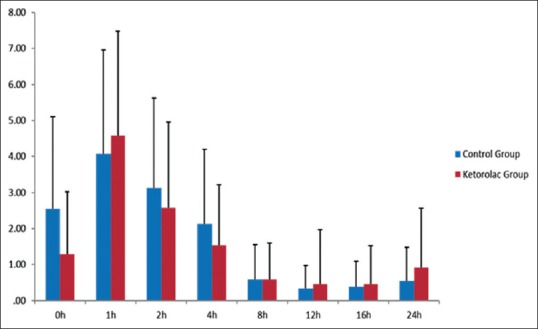
Visual analog scale scores in the two groups during the 24 h after surgery (mean ± standard deviation). There were no statistically significant differences between groups (P > 0.05). There was a significant effect of time in pain scores for each group separately (P < 0.001)
There were no significant differences between groups in cumulative consumption of tramadol at any time point during the first postoperative 24 h (P > 0.05). The effect of time on the total consumption of tramadol through PCA in the entire immediate postoperative period was statistically significant in both groups (P < 0.001) [Figure 2].
Figure 2.
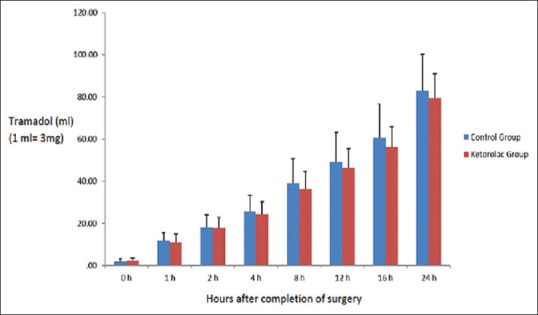
Cumulative patient-controlled analgesia tramadol consumption in both groups during the 24 h after surgery (mean ± standard deviation). There were no significant differences between the two groups (P > 0.05). The effect of time on total tramadol consumption in the postoperative period was statistically significant (P < 0.001)
The amount of incremental postoperative doses of tramadol consumption in bolus from the PCA was similar in the two groups. There were no statistically significant differences among groups at any time point (P > 0.05). The need of additional boluses of tramadol over the basal infusion rate of the PCA was higher in the control group at all time points, except immediately after arrival at the PACU. The difference between groups in the total amount of bolus supplements of tramadol needed over the 24 h was not statistically significant (P = 0.106) [Figure 3].
Figure 3.
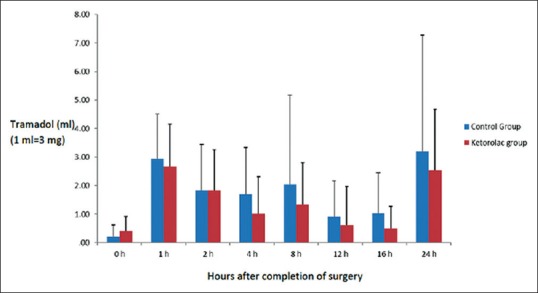
Incremental patient-controlled analgesia tramadol consumption in bolus in both groups during the 24 h after surgery (mean ± standard deviation). There were no statistically significant differences among groups (P > 0.05). The effect of time on tramadol consumption in the postoperative period was statistically significant (P < 0.001)
No differences in patient satisfaction were observed between the groups (P = 0.848). The majority of patients evaluated their pain control as very good over the 24 h after the operation.
Side effects seen in the ketorolac group were nausea (seven patients) and vomiting (five patients). In the control group, they were also nausea (five patients) and vomiting (four patients). These differences were not statistically significant (P = 0.481). No patients experienced side effects defined as serious [Table 3].
Table 3.
Adverse effects

The time to first requested rescue analgesia was 66.25 ± 38.61 min in the ketorolac group (eight patients) and 65 ± 28.86 min in the control group (seven patients). The difference was not statistically significant (P = 0.765).
When evaluating the hemodynamic parameters as an indirect measure of pain, we found the following results. The systolic BP at all time points during the postoperative 24 h was very similar between both groups, with no statistically significant differences (P > 0.05), except at 2 h (P = 0.02) and 4 h (P = 0.045). These differences were not clinically significant. There was an increase in systolic BP on arrival at the PACU, with a progressive decrease over the 24 h until final stabilization. The diastolic BP was similar between both groups, with no statistically significant differences, except at 4 h, being higher in the ketorolac group (P = 0.013). The effect of time in systolic and diastolic BP was significant in both groups (P < 0.001). The respiratory rate showed no differences between groups, except at 8 h (P = 0.017), 16 h (P = 0.011), and 24 h (P = 0.049). These differences did not appear to be clinically significant. The respiratory rate was very stable throughout the entire postoperative period. There were no significant differences between the groups in heart rate at any time point (P > 0.05). The effect of time on heart rate through the postoperative period was statistically significant (P < 0.05).
Discussion
Direct surgical trauma may not be the only cause of pain in the postoperative period: the human body can magnify pain, resulting in pain hypersensitivity[2] causing an exaggerated answer to noxious stimuli peripherally and centrally in the spinal cord.[36] Supported on the aforementioned, the idea of preemptive analgesia has been unfolded. Most animal studies have exhibited a preemptive analgesic effect.[37] Nonetheless, in clinical studies, this effect was contemplated only in a few cases.[38,39]
Preoperative administration of NSAIDs reduces the responsiveness of pain nerves to endogenous inflammatory factors and inhibits sensitization of the central nervous systems (CNSs),[1,40] appearing to have a peripheral and central function in the prevention of postoperative pain.[41,42,43]
Ketorolac has several other properties that make it an appealing candidate as a preemptive analgesic. These comprise its moderate potency (analogous to morphine in some studies[44]), convenience of administration IV or intramuscularly, lack of acute tolerance,[9] and lack of considerable cardiorespiratory or CNS side effects.[1] Its two primary unfavorable effects, interference with renal and platelet function, should be insignificant with cautious patient selection.[36]
The intention of our study was to assess whether ketorolac has any preemptive analgesic action in the management of postoperative pain.[45]
In our study, no considerable difference was perceived in the pain intensity among groups during the first postoperative 24 h. No considerable decrease in the quantity of opioid intake happened in the ketorolac group. The hemodynamics persisted almost unchanged in all patients throughout the study period, without statistically significant differences among groups, except at 2 and 4 h for the systolic BP, 4 h for the diastolic BP, and at 8, 16, and 24 h for the respiratory rate. In addition, the time to first rescue morphine intake was not statistically different between the groups.
Most patients reported adequate analgesia in the immediate postoperative period, as reflected in the pain scores that were low in both groups. The upper limits of VAS were 3–4.5; these scores are generally accepted as adequate.
There are several possible explanations for the absence of a noticeable preemptive analgesic advantage of ketorolac in our study. First, the 30 mg dose of ketorolac may have been inadequate. We selected this dosage based on published recommendations;[16,46,47,48,49] in Great Britain, the recommended dose is only 10 mg, nevertheless, doses of 60 mg have been used in the United States.[50]
Rogers et al.[51] showed lower opioid usage after abdominal hysterectomy in patients who received 10 mg of ketorolac IV before surgery compared with given after skin closure. This action was only noted 2 h postoperatively, possibly because of the low dose of ketorolac used. In contraposition, Fletcher et al.[31] examined the effects of 60 mg of IV ketorolac injected before induction in patients undergoing total hip replacement in comparison to administration during skin closure. Those receiving ketorolac prior to induction had lower VAS scores and diminished opioid usage throughout the first 6 h postoperatively.[36] Putland and McCluskey[52] showed that IV tramadol (100 mg) was a more effective preemptive analgesic than ketorolac (10 mg) during laparoscopic surgery.[45] Therefore, we chose a dose of 30 mg ketorolac compared with a dose of 10 mg.[53] Studies on the dose/effect relation of ketorolac in postoperative pain relief appear warranted to improve knowledge on the optimal dose.[54]
Only one dose of ketorolac was used. Woolf and Chong[2] suggested that chemical mediators released by the inflammatory response to tissue trauma produce continuing nociceptor excitement until wound healing is accomplished and would overshadow preemptive effects; thus, optimal “preemptive” analgesia may involve continuing ketorolac into the postoperative period.[36]
The use of opioids intraoperatively may have obscured any potential difference between the groups by providing good analgesia in both groups. The opioid analgesia may have avoided sensitization of spinal neurons that might have submerged the short-lived, preemptive effect of ketorolac. We used a standard regimen of perioperative analgesia to imitate the common clinical discipline at our institution.[36]
In continuation, the use of PCA has been criticized as a method of assessing outcome measures in analgesia studies.[11] Depending on opioid-sparing effects as the main suggestion of improved analgesia postoperatively is a potential concern because many other components may influence PCA opioid usage, including atmosphere, anxiety, expectations of recovery, awareness of support,[11,55] and the amount of the demand dose. Accordingly, total opioid consumption may not actually represent pain intensity.[5]
Another likelihood for the negative result is a possible preemptive analgesic effect of nitrous oxide prior to skin incision.[56,57] All our patients were given nitrous oxide before the skin incision. This might have concealed any effect of ketorolac.[5]
The severity of the surgical trauma could be another factor affecting the results. There are studies that have shown a reduced need for postoperative analgesics when anesthesia, for outpatient laparoscopy, was supplemented with NSAIDs.[58,59] However, in the study by Hovorka et al.,[59] the effect was seen only in patients undergoing diagnostic procedures, albeit it was not effective enough to avoid pain after laparoscopic tubal ligation. In a recent study in adults undergoing elective tonsillectomy, only minimal effects on postoperative pain could be found.[54] Cepeda et al.[60] found that if pain is severe, the odds of having pain relief is lower than when pain is moderate. Accordingly, differences in the baseline pain intensity among studies that assessed NSAIDs could answer the discrepancy.[60]
It is feasible that any preemptive effect of NSAIDs is too small to be of provable clinical magnitude.[51,61] The function of PGs in the pain pathway denotes that NSAIDs might diminish, rather than abolish, the afferent nociceptive input to the spinal cord. Such attenuation may not be enough to completely avoid sensitization, which may account for the lack of preemptive benefit. In addition, PGs are only one of a number of chemicals comprised in nociception and sensitization.[5]
From an assessment of the overall results acquired in our study, we could not establish any considerable preemptive analgesic advantage of ketorolac given preoperatively when compared with placebo. Thus, further comparative and controlled studies of the effects of higher doses in larger study sizes are needed before final recommendations can be made.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Wang Y, Zhang HB, Xia B, Wang GM, Zhang MY. Preemptive analgesic effects of flurbiprofen axetil in patients undergoing radical resection of esophageal carcinoma via the left thoracic approach. Chin Med J (Engl) 2012;125:579–82. [PubMed] [Google Scholar]
- 2.Woolf CJ, Chong MS. Preemptive analgesia – Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 3.Reuben SS, Buvanendran A. Preventing the development of chronic pain after orthopaedic surgery with preventive multimodal analgesic techniques. J Bone Joint Surg Am. 2007;89:1343–58. doi: 10.2106/JBJS.F.00906. [DOI] [PubMed] [Google Scholar]
- 4.Grifka J, Enz R, Zink J, Hugot JL, Kreiss A, Arulmani U, et al. Preemptive versus postoperative lumiracoxib for analgesia in ambulatory arthroscopic knee surgery. J Pain Res. 2008;1:27–34. doi: 10.2147/jpr.s3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gabbott DA, Cohen AM, Mayor AH, Niemiro LA, Thomas TA. The influence of timing of ketorolac administration on post-operative analgesic requirements following total abdominal hysterectomy. Eur J Anaesthesiol. 1997;14:610–5. doi: 10.1046/j.1365-2346.1994.00190.x. [DOI] [PubMed] [Google Scholar]
- 6.Woolf CJ. Bond MR, Charlton JE, Woolf CJ. Proceedings of the VIth World Congress on Pain. Amsterdam: Elsevier Science Publishers BV; 1991. Central mechanisms of acute pain; pp. 25–34. [Google Scholar]
- 7.McQuay HJ, Dickenson AH. Implications of nervous system plasticity for pain management. Anaesthesia. 1990;45:101–2. doi: 10.1111/j.1365-2044.1990.tb14270.x. [DOI] [PubMed] [Google Scholar]
- 8.Dahl JB, Kehlet H. The value of pre-emptive analgesia in the treatment of postoperative pain. Br J Anaesth. 1993;70:434–9. doi: 10.1093/bja/70.4.434. [DOI] [PubMed] [Google Scholar]
- 9.McQuay HJ. Pre-emptive analgesia. Br J Anaesth. 1992;69:1–3. doi: 10.1093/bja/69.1.1. [DOI] [PubMed] [Google Scholar]
- 10.Bush DJ. Pre-emptive analgesia. BMJ. 1993;306:285–6. doi: 10.1136/bmj.306.6873.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kissin I. Preemptive analgesia. Why its effect is not always obvious. Anesthesiology. 1996;84:1015–9. doi: 10.1097/00000542-199605000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Rockemann MG, Seeling W, Bischof C, Börstinghaus D, Steffen P, Georgieff M. Prophylactic use of epidural mepivacaine/morphine, systemic diclofenac, and metamizole reduces postoperative morphine consumption after major abdominal surgery. Anesthesiology. 1996;84:1027–34. doi: 10.1097/00000542-199605000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Katz J, Clairoux M, Kavanagh BP, Roger S, Nierenberg H, Redahan C, et al. Pre-emptive lumbar epidural anaesthesia reduces postoperative pain and patient-controlled morphine consumption after lower abdominal surgery. Pain. 1994;59:395–403. doi: 10.1016/0304-3959(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 14.Buvanendran A, Kroin JS, Tuman KJ, Lubenow TR, Elmofty D, Moric M, et al. Effects of perioperative administration of a selective cyclooxygenase 2 inhibitor on pain management and recovery of function after knee replacement: A randomized controlled trial. JAMA. 2003;290:2411–8. doi: 10.1001/jama.290.18.2411. [DOI] [PubMed] [Google Scholar]
- 15.Likar R, Krumpholz R, Mathiaschitz K, Pipam W, Burtscher M, Ozegovic G, et al. The preemptive action of ketoprofen. Randomized, double-blind study with gynecologic operations. Anaesthesist. 1997;46:186–90. doi: 10.1007/s001010050389. [DOI] [PubMed] [Google Scholar]
- 16.Murphy DF, Medley C. Preoperative indomethacin for pain relief after thoracotomy: Comparison with postoperative indomethacin. Br J Anaesth. 1993;70:298–300. doi: 10.1093/bja/70.3.298. [DOI] [PubMed] [Google Scholar]
- 17.Gustafsson I, Nyström E, Quiding H. Effect of preoperative paracetamol on pain after oral surgery. Eur J Clin Pharmacol. 1983;24:63–5. doi: 10.1007/BF00613928. [DOI] [PubMed] [Google Scholar]
- 18.Millar AY, Mansfield MD, Kinsella J. Influence of timing of morphine administration on postoperative pain and analgesic consumption. Br J Anaesth. 1998;81:373–6. doi: 10.1093/bja/81.3.373. [DOI] [PubMed] [Google Scholar]
- 19.Wordliczek J, Banach M, Garlicki J, Jakowicka-Wordliczek J, Dobrogowski J. Influence of pre- or intraoperational use of tramadol (preemptive or preventive analgesia) on tramadol requirement in the early postoperative period. Pol J Pharmacol. 2002;54:693–7. [PubMed] [Google Scholar]
- 20.Ko SH, Lim HR, Kim DC, Han YJ, Choe H, Song HS. Magnesium sulfate does not reduce postoperative analgesic requirements. Anesthesiology. 2001;95:640–6. doi: 10.1097/00000542-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Dorazil-Dudzik M, Mika J, Schafer MK, Li Y, Obara I, Wordliczek J, et al. The effects of local pentoxifylline and propentofylline treatment on formalin-induced pain and tumor necrosis factor-alpha messenger RNA levels in the inflamed tissue of the rat paw. Anesth Analg. 2004;98:1566–73. doi: 10.1213/01.ANE.0000113235.88534.48. [DOI] [PubMed] [Google Scholar]
- 22.Wordliczek J, Szczepanik AM, Banach M, Turchan J, Zembala M, Siedlar M, et al. The effect of pentoxifiline on post-injury hyperalgesia in rats and postoperative pain in patients. Life Sci. 2000;66:1155–64. doi: 10.1016/s0024-3205(00)00419-7. [DOI] [PubMed] [Google Scholar]
- 23.Dahl V, Ernoe PE, Steen T, Raeder JC, White PF. Does ketamine have preemptive effects in women undergoing abdominal hysterectomy procedures? Anesth Analg. 2000;90:1419–22. doi: 10.1097/00000539-200006000-00031. [DOI] [PubMed] [Google Scholar]
- 24.Wordliczek J, Banach M, Dorazil M, Przewlocka B. Influence of doxepin used in preemptive analgesia on the nociception in the perioperative period. Experimental and clinical study. Pol J Pharmacol. 2001;53:253–61. [PubMed] [Google Scholar]
- 25.Wnek W, Zajaczkowska R, Wordliczek J, Dobrogowski J, Korbut R. Influence of pre-operative ketoprofen administration (preemptive analgesia) on analgesic requirement and the level of prostaglandins in the early postoperative period. Pol J Pharmacol. 2004;56:547–52. [PubMed] [Google Scholar]
- 26.Rang HP, Bevan S, Dray A. Chemical activation of nociceptive peripheral neurones. Br Med Bull. 1991;47:534–48. doi: 10.1093/oxfordjournals.bmb.a072491. [DOI] [PubMed] [Google Scholar]
- 27.Rorarius MG, Baer GA. Non-steroidal anti-inflammatory drugs for postoperative pain relief. Curr Opin Anesthesiol. 1994;7:358–62. [Google Scholar]
- 28.Malmberg AB, Yaksh TL. Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther. 1992;263:136–46. [PubMed] [Google Scholar]
- 29.Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–9. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 30.Katz J. Pre-emptive analgesia: Importance of timing. Can J Anaesth. 2001;48:105–14. doi: 10.1007/BF03019721. [DOI] [PubMed] [Google Scholar]
- 31.Fletcher D, Zetlaoui P, Monin S, Bombart M, Samii K. Influence of timing on the analgesic effect of intravenous ketorolac after orthopedic surgery. Pain. 1995;61:291–7. doi: 10.1016/0304-3959(94)00184-G. [DOI] [PubMed] [Google Scholar]
- 32.Björkman R, Hallman KM, Hedner J, Hedner T, Henning M. Nonsteroidal antiinflammatory drug modulation of behavioral responses to intrathecal N-methyl-D-aspartate, but not to substance P and amino-methyl-isoxazole-propionic acid in the rat. J Clin Pharmacol. 1996;36(12 Suppl):20S–6S. [PubMed] [Google Scholar]
- 33.Masue T, Dohi S, Asano T, Shimonaka H. Spinal antinociceptive effect of epidural nonsteroidal antiinflammatory drugs on nitric oxide-induced hyperalgesia in rats. Anesthesiology. 1999;91:198–206. doi: 10.1097/00000542-199907000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Lee JI, Burckart GJ. Nuclear factor kappa B: Important transcription factor and therapeutic target. J Clin Pharmacol. 1998;38:981–93. doi: 10.1177/009127009803801101. [DOI] [PubMed] [Google Scholar]
- 35.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: An update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002;68-69:433–55. doi: 10.1016/s0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 36.Norman PH, Daley MD, Lindsey RW. Preemptive analgesic effects of ketorolac in ankle fracture surgery. Anesthesiology. 2001;94:599–603. doi: 10.1097/00000542-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Kissin I. Preemptive analgesia. Anesthesiology. 2000;93:1138–43. doi: 10.1097/00000542-200010000-00040. [DOI] [PubMed] [Google Scholar]
- 38.Katz J, Kavanagh BP, Sandler AN, Nierenberg H, Boylan JF, Friedlander M, et al. Preemptive analgesia. Clinical evidence of neuroplasticity contributing to postoperative pain. Anesthesiology. 1992;77:439–46. doi: 10.1097/00000542-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: The role of timing of analgesia. Anesthesiology. 2002;96:725–41. doi: 10.1097/00000542-200203000-00032. [DOI] [PubMed] [Google Scholar]
- 40.Lu JC, Zhang XF, Liu C. Effect of preemptive analgesia with flurbiprofen axetil on patient-controlled intravenous analgesia with tramadol in patients undergoing postburn plastic surgery. J South Med Univ Chin. 2009;29:1255–6. [PubMed] [Google Scholar]
- 41.Dahl JB, Kehlet H. Non-steroidal anti-inflammatory drugs: Rationale for use in severe postoperative pain. Br J Anaesth. 1991;66:703–12. doi: 10.1093/bja/66.6.703. [DOI] [PubMed] [Google Scholar]
- 42.Rosenblum M, Weller RS, Conard PL, Falvey EA, Gross JB. Ibuprofen provides longer lasting analgesia than fentanyl after laparoscopic surgery. Anesth Analg. 1991;73:255–9. doi: 10.1213/00000539-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 43.O’Hanlon JJ, Beers H, Huss BK, Milligan KR. A comparison of the effect of intramuscular diclofenac, ketorolac or piroxicam on post-operative pain following laparoscopy. Eur J Anaesthesiol. 1996;13:404–7. doi: 10.1046/j.1365-2346.1996.d01-365.x. [DOI] [PubMed] [Google Scholar]
- 44.Yee JP, Koshiver JE, Allbon C, Brown CR. Comparison of intramuscular ketorolac tromethamine and morphine sulfate for analgesia of pain after major surgery. Pharmacotherapy. 1986;6:253–61. doi: 10.1002/j.1875-9114.1986.tb03485.x. [DOI] [PubMed] [Google Scholar]
- 45.Gutta R, Koehn CR, James LE. Does ketorolac have a preemptive analgesic effect. A randomized, double-blind, control study? J Oral Maxillofac Surg. 2013;71:2029–34. doi: 10.1016/j.joms.2013.06.220. [DOI] [PubMed] [Google Scholar]
- 46.Ding Y, Fredman B, White PF. Use of ketorolac and fentanyl during outpatient gynecologic surgery. Anesth Analg. 1993;77:205–10. doi: 10.1213/00000539-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Korttila K. Recovery period and discharge. In: White P, editor. Outpatient Anaesthesia. New York: Churchill Livingstone; 1990. pp. 369–96. [Google Scholar]
- 48.Ready LB, Brown CR, Stahlgren LH, Egan KJ, Ross B, Wild L, et al. Evaluation of intravenous ketorolac administered by bolus or infusion for treatment of postoperative pain. A double-blind, placebo-controlled, multicenter study. Anesthesiology. 1994;80:1277–86. doi: 10.1097/00000542-199406000-00015. [DOI] [PubMed] [Google Scholar]
- 49.Sandin R, Sternlo JE, Stam H, Brodd B, Björkman R. Diclofenac for pain relief after arthroscopy: A comparison of early and delayed treatment. Acta Anaesthesiol Scand. 1993;37:747–50. doi: 10.1111/j.1399-6576.1993.tb03802.x. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Ding Y, White PF, Feinstein R, Shear JM. Effects of ketorolac on postoperative analgesia and ventilatory function after laparoscopic cholecystectomy. Anesth Analg. 1993;76:1061–6. doi: 10.1213/00000539-199305000-00026. [DOI] [PubMed] [Google Scholar]
- 51.Rogers JE, Fleming BG, Macintosh KC, Johnston B, Morgan-Hughes JO. Effect of timing of ketorolac administration on patient-controlled opioid use. Br J Anaesth. 1995;75:15–8. doi: 10.1093/bja/75.1.15. [DOI] [PubMed] [Google Scholar]
- 52.Putland AJ, McCluskey A. The analgesic efficacy of tramadol versus ketorolac in day-case laparoscopic sterilisation. Anaesthesia. 1999;54:382–5. doi: 10.1046/j.1365-2044.1999.00739.x. [DOI] [PubMed] [Google Scholar]
- 53.Fricke JR, Jr, Angelocci D, Fox K, McHugh D, Bynum L, Yee JP. Comparison of the efficacy and safety of ketorolac and meperidine in the relief of dental pain. J Clin Pharmacol. 1992;32:376–84. doi: 10.1002/j.1552-4604.1992.tb03850.x. [DOI] [PubMed] [Google Scholar]
- 54.Jakobsson J, Rane K, Davidson S. Intramuscular NSAIDS reduce post-operative pain after minor outpatient anaesthesia. Eur J Anaesthesiol. 1996;13:67–71. doi: 10.1097/00003643-199601000-00012. [DOI] [PubMed] [Google Scholar]
- 55.Jamison RN, Taft K, O’Hara JP, Ferrante FM. Psychosocial and pharmacologic predictors of satisfaction with intravenous patient-controlled analgesia. Anesth Analg. 1993;77:121–5. [PubMed] [Google Scholar]
- 56.Goto T, Marota JJ, Crosby G. Nitrous oxide induces preemptive analgesia in the rat that is antagonized by halothane. Anesthesiology. 1994;80:409–16. doi: 10.1097/00000542-199402000-00021. [DOI] [PubMed] [Google Scholar]
- 57.O’Connor TC, Abram SE. Inhibition of nociception-induced spinal sensitization by anesthetic agents. Anesthesiology. 1995;82:259–66. doi: 10.1097/00000542-199501000-00031. [DOI] [PubMed] [Google Scholar]
- 58.Ding Y, White PF. Comparative effects of ketorolac, dezocine, and fentanyl as adjuvants during outpatient anesthesia. Anesth Analg. 1992;75:566–71. doi: 10.1213/00000539-199210000-00018. [DOI] [PubMed] [Google Scholar]
- 59.Hovorka J, Kallela H, Korttila K. Effect of intravenous diclofenac on pain and recovery profile after day-case laparoscopy. Eur J Anaesthesiol. 1993;10:105–8. [PubMed] [Google Scholar]
- 60.Cepeda MS, Carr DB, Miranda N, Diaz A, Silva C, Morales O. Comparison of morphine, ketorolac, and their combination for postoperative pain: Results from a large, randomized, double-blind trial. Anesthesiology. 2005;103:1225–32. doi: 10.1097/00000542-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 61.Parke TJ, Lowson SM, Uncles DR, Daughtery MO, Sitzman BT. Pre-emptive versus post-surgical administration of ketorolac for hysterectomy. Eur J Anaesthesiol. 1995;12:549–53. [PubMed] [Google Scholar]


