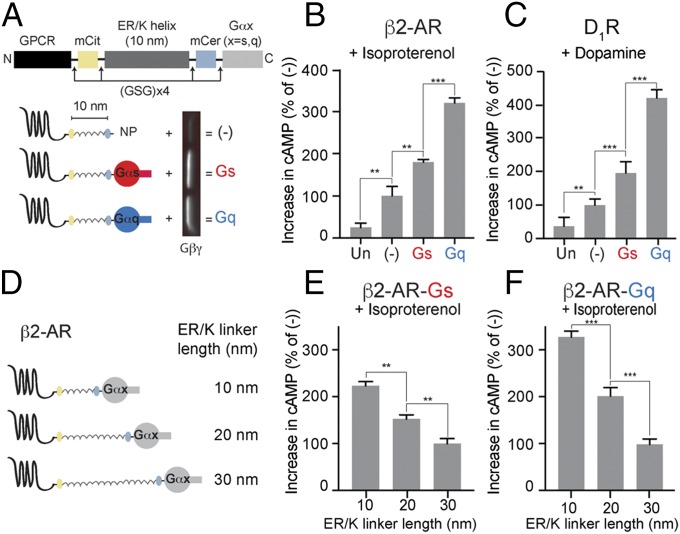
Fig. 1.
Effect of tethered Gα subunits on signaling via Gs-coupled receptors. (A) Schematics of GPCR G-protein sensors used here. The GPCR (β2-AR or D1-R), mCitrine, 10-nm ER/K linker, mCerulean, and Gα subunit (Gαs, red, or Gαq, blue) are expressed as a single polypeptide, separated from each other by Gly–Ser–Gly (GSG) × 4 linkers. Sensors that terminated at a Gly–Ser–Gly × 4 peptide without Gα (NP, no peptide at the end) are indicated as (−) and were used as controls. Western blot of membranes purified from sensor-expressing cells, probed with Gβ antibody, reveal interaction of tethered Gα subunits with endogenous Gβγ. (B and C) Increase in cAMP levels between buffer-treated and agonist-treated (B, 10 μM isoproterenol; C, 10 μM dopamine) HEK293T cells expressing equivalent amount of GPCR G-protein sensors. Gαq tethered to the receptors via 10-nm ER/K linker exhibits the greatest increase in cAMP, a phenomenon we term GPCR priming. (D) Schematics of β2-AR sensors tethered to Gα subunits through ER/K linker length varied sequentially from 10 to 30 nm. (E and F) Effect of linker length used for tethering Gα subunit to β2-AR on cAMP levels between isoproterenol (10 μM) and buffer-treated HEK293T cells expressing equivalent levels of sensors. (B, C, E, and F) Values are mean ± SEM from n ≥ 10 observations over at least three independent experiments. **P < 0.01, ***P < 0.005 by unpaired t test.