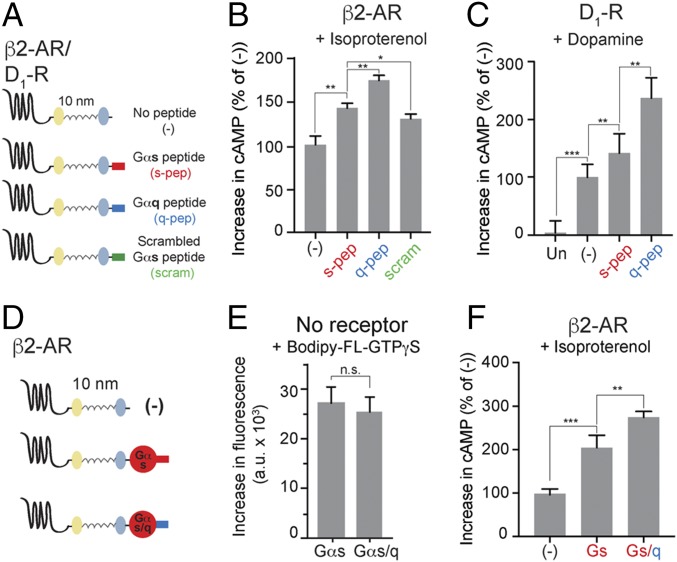
Fig. 2.
Tethered Gα C terminus peptide is sufficient for GPCR priming. (A) Schematics of the peptide sensors used. The GPCR (β2-AR or D1-R), mCitrine, 10-nm ER/K linker, mCerulean, and α5 peptide from the Gα C terminus of either Gαs (s-pep, red) or Gαq (q-pep, blue) or scrambled sequence from Gαs peptide (scrambled, scram, green) are expressed as a single polypeptide, separated from each other by Gly–Ser–Gly (GSG) × 4 linkers. (B and C) Increase in cAMP levels between buffer-treated and agonist-treated (B, 10 μM isoproterenol; C, 10 μM dopamine) HEK293T cells expressing equivalent amount of peptide sensors. Tethered q-pep exhibits the greatest increase in cAMP. (D) Chimeric Gαs/q constructed by swapping the C terminus α5 peptide of Gαs with the corresponding peptide from Gαq. Comparison of sensors tethering β2-AR to Gαs and chimeric Gαs/q. (E) GTP-binding ability of Gαs and Gαs/q. Incorporation of BODIPY-FL–GTPγS into Gα subunits measured as an increase in fluorescence between BODIPY-FL–GTPγS alone, and BODIPY-FL–GTPγS with indicated Gα subunit. (F) Increase in cAMP levels between buffer-treated and isoproterenol-treated (10 μM) HEK293T cells expressing equivalent amount of indicated sensors. Tethered chimeric Gαs/q exhibits the greatest increase in cAMP. (B, C, and F) Values are mean ± SEM from n ≥ 10 observations over at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.005 by unpaired t test.