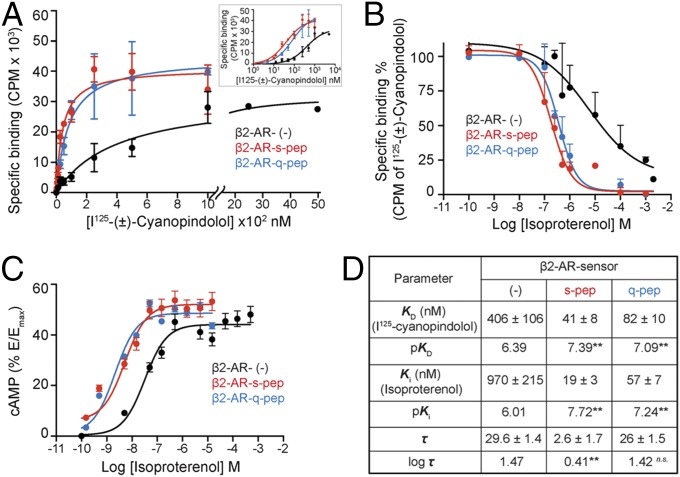
Fig. 4.
Gα C terminus peptides increase the potency of cAMP response. (A) Radioligand binding to purified membranes. Saturation binding of (±)-[125I]iodocyanopindolol to 10 μg of purified membranes from cells expressing β2-AR sensors was measured from bound radioactivity as a function of increasing radioligand concentration and used to calculate the equilibrium dissociation constant (KD). Membranes containing β2-AR control sensors require greater radioligand to display saturation (displayed with broken axis). (Inset) Radioligand binding data represented on a logarithmic x axis. (B) Competition of radioligand binding to β2-AR sensors by isoproterenol. Purified membranes containing 20 fmol of indicated β2-AR sensors were incubated with increasing isoproterenol in the presence of excess (±)-[125I]iodocyanopindolol (peptide sensors, 500 nM; control sensor, 2 μM). Bound radioactivity was measured as a function of increasing isoproterenol concentration to estimate equilibrium dissociation constant (Ki). (C) cAMP accumulation in HEK293T cells expressing equivalent amounts of β2-AR sensors. Cells expressing control or peptide sensors were stimulated with varying concentration of isoproterenol (0.1 nM to 1 mM) and the cAMP response measured. Increase in cAMP was expressed as a percentage of the cAMP response from forskolin stimulation (Emax), for each sensor. Resulting concentration–response curves were fitted to the operational model to estimate receptor efficacy (log τ), with Ki (from B) as a constraining parameter. (D) Values are mean ± SEM obtained by analysis of the three independent experiments. **P < 0.01, by unpaired t test, comparing values for each peptide to the control sensor.