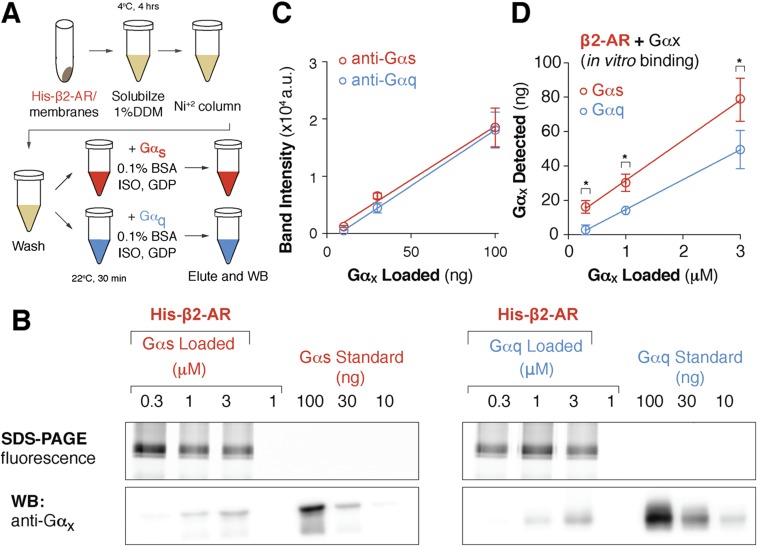
Fig. S1.
β2-AR binds to cognate Gαs more strongly than Gαq subunit. (A) Schematic of the coimmunoprecipitation assay used to assess binding between purified Gαs or Gαq subunit and His-tagged β2-AR control sensors bound to Ni2+ resin. (B) Eluted β2-AR and Gαx complexes were separated on SDS/PAGE. (Top) Gels were scanned for mCitrine fluorescence to verify equivalent loading. (Bottom) Representative Western blot of Gαs and Gαq coimmunoprecipitation. (Bottom Left) Total Gαx was detected via anti-Gαs and (Bottom Right) anti-Gαq antibodies. (B and C) Antibody sensitivity was assessed using purified Gαx standards (anti-Gαs R2 = 0.99; anti-Gαq R2 = 1.00). (D) Purified Gαx standards were used to quantify total Gαx bound to β2-AR. Total Gαx detected (in nanograms) compared with the equivalent concentration of Gαx (in micromolar concentration) added in the β2-AR-Gαx binding assay. Data are mean ± SEM from at least three independent experiments. *P < 0.05, Student’s unpaired t test.