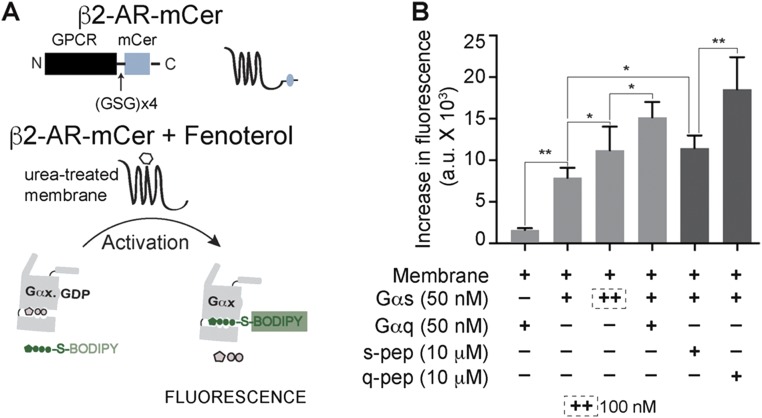
Fig. S4.
G-protein activation profile is preserved in absence of the ER/K linker. (A) Schematic representation of the β2-AR–mCer fusion protein (β2-AR fused to mCerulean without any ER/K linker). Gα subunit activation in vitro is measured by increase in fluorescence of BODIPY-FL–GTPγS. Activation is triggered by addition of fenoterol to urea-treated membranes isolated from cells expressing β2-AR–mCer fusion that lacks the ER/K linker, compared with β2-AR control sensor containing an ER/K linker. (B) Effect of Gα proteins (+, 50 nM; ++, 100 nM) and soluble Gα C terminus peptides (10 μM) on the in vitro activation of Gαs by fenoterol treatment of β2-AR–mCer. Gαq causes synergistic activation of Gασ. s-pep and q-pep increase the activation of Gασ, with q-pep showing an augmented increase. Values are mean ± SEM from n ≥ 5 observations from three independent experiments. *P < 0.05, **P < 0.01 by unpaired t test.