Significance
Silent biosynthetic gene clusters represent fertile grounds for the discovery of new small molecules, and a number of approaches have been developed for activating them. Their regulation, however, has received far less attention, especially in Gram-negative bacteria. Here, we show that a LysR-type transcriptional regulator, which we name scmR, acts as a gatekeeper of secondary metabolism and virulence in the model organism Burkholderia thailandensis E264. Specifically, genetic, chemical, and “omics”-based approaches unveil ScmR as a master regulator that interacts with pathway-specific transcriptional regulators to control, and primarily suppress, secondary metabolism. Because this gene is conserved in other Burkholderia species, our results suggest that scmR-mediated control of secondary metabolism and virulence may be common within this genus and the Pseudomallei-group pathogens.
Keywords: biosynthetic gene clusters, natural products, Burkholderia thailandensis, regulation, virulence
Abstract
Bacteria produce a diverse array of secondary metabolites that have been invaluable in the clinic and in research. These metabolites are synthesized by dedicated biosynthetic gene clusters (BGCs), which assemble architecturally complex molecules from simple building blocks. The majority of BGCs in a given bacterium are not expressed under normal laboratory growth conditions, and our understanding of how they are silenced is in its infancy. Here, we have addressed this question in the Gram-negative model bacterium Burkholderia thailandensis E264 using genetic, transcriptomic, metabolomic, and chemical approaches. We report that a previously unknown, quorum-sensing-controlled LysR-type transcriptional regulator, which we name ScmR (for secondary metabolite regulator), serves as a global gatekeeper of secondary metabolism and a repressor of numerous BGCs. Transcriptionally, we find that 13 of the 20 BGCs in B. thailandensis are significantly (threefold or more) up- or down-regulated in a scmR deletion mutant (ΔscmR). Metabolically, the ΔscmR strain displays a hyperactive phenotype relative to wild type and overproduces a number of compound families by 18- to 210-fold, including the silent virulence factor malleilactone. Accordingly, the ΔscmR mutant is hypervirulent both in vitro and in a Caenorhabditis elegans model in vivo. Aside from secondary metabolism, ScmR also represses biofilm formation and transcriptionally activates ATP synthesis and stress response. Collectively, our data suggest that ScmR is a pleiotropic regulator of secondary metabolism, virulence, biofilm formation, and other stationary phase processes. A model for how the interplay of ScmR with pathway-specific transcriptional regulators coordinately silences virulence factor production is proposed.
Bacterial secondary metabolites represent a dominant source of pharmaceutical compounds and comprise some of our most celebrated cures (1, 2). The genes responsible for biosynthesizing these complex molecules, such as vancomycin or bleomycin, are often clustered in bacterial chromosomes (3). The corresponding biosynthetic gene clusters (BGCs) can be identified bioinformatically and serve as a marker or indicator of a bacterium’s capacity for secondary metabolite production. Genomically speaking, the most prolific bacterial producers belong to the actinomycetes, myxobacteria, and some Proteobacteria, like the genus Burkholderia (3–6). Members within these families typically contain >20 BGCs; some actinomycetes harbor as many as 50. However, the majority of these BGCs do not give rise to detectable levels of secondary metabolites under normal laboratory conditions, prompting terms such as “silent” or “cryptic” to describe them. In general, silent BGCs outnumber constitutively active ones by a factor of 5–10, suggesting that the microbial small molecules discovered thus far merely represent the tip of the iceberg.
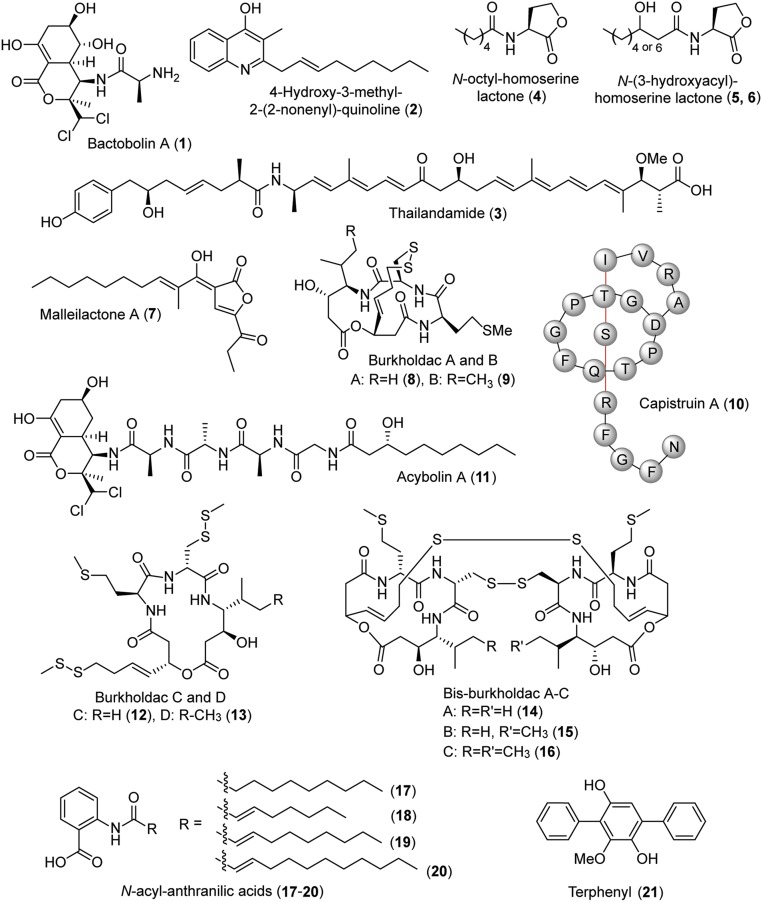
Burkholderia thailandensis E264 is a saprophytic soil organism, isolated from rice fields in Thailand (7). It is an avirulent model for its pathogenic relatives, Burkholderia pseudomallei and Burkholderia mallei, the causative agents of the emerging disease meliodosis and glanders in equines, respectively (8, 9). The genome of B. thailandensis E264 harbors 23 BGCs, although only six of these—the bactobolin (bta; 1; Fig. 1) (10, 11), 4-hydroxy-3-methyl-2-alkylquinoline (hmq; 2) (12), and thailandamide (tha; 3) gene clusters (13), and three N-acyl homoserine lactones (AHLs; 4–6) (11, 14) —give rise to appreciable amounts of product during normal laboratory growth (Fig. 1) (5). Included in the store of silent or lowly expressed BGCs are the malleilactone (mal; 7) (15, 16), burkholdac (bhc; 8 and 9) (17), and capistruin (cap; 10) gene clusters (Fig. 1) (18). By using a promoter insertion strategy, the Brady and Hertweck groups were able to induce and structurally elucidate malleilactone, the valence tautomer of which is referred to as burkholderic acid (7) (15, 16). The Brady group further showed that the mal cluster is required for toxicity in Caenorhabditis elegans, suggesting that malleilactone is a silent virulence factor against worms. The product of the bhc cluster, burkholdac (8 and 9), was identified by overexpression of a pathway-specific regulator (17). It is a hybrid polyketide/nonribosomal peptide and a potent inhibitor of some histone deacetylases (HDACs). Finally, the lasso peptide capistruin was identified by heterologous expression in Escherichia coli and by subjecting B. thailandensis to heat stress (18). It is not produced under normal growth conditions. Collectively, these considerable efforts have provided the products of some of the silent gene clusters in B. thailandensis, although their endogenous activation remains unknown.
Fig. 1.
Selected secondary metabolites produced by B. thailandensis E264. Compounds 1–11 and 21 were identified previously (see text). Compounds 12–20 were elucidated in this study.
The regulation of these and other silent gene clusters is an area of immense interest. B. thailandensis E264 contains three acyl homoserine lactone-dependent quorum sensing (QS) systems, with which it regulates expression of ∼300 genes as a function of cell density (19). These consist of LuxI/LuxR synthase/response regulator pairs, which are termed BtaI1/BtaR1, BtaI2/BtaR2, and BtaI3/BtaR3. RNA-sequencing (RNA-seq) experiments by the Greenberg laboratory have shown that a number of BGCs are under QS control. Some clusters, notably bta, are strongly activated by QS, and, as a result, the ensuing secondary metabolite is easily detected during stationary phase growth (19). The regulation of other BGCs is more complex because they may be repressed by a specific BtaI/BtaR pair, while activated by others. These results are consistent with regulation of secondary metabolism in other Proteobacteria, which is known to be complex as well. In perhaps the best-studied case, Pseudomonas aeruginosa, transcriptional regulators other than LuxRs also play important roles (20). Specifically, LysR-type transcriptional regulators (LTTRs), such as mvfR, are key in the temporal control of secondary metabolite production in P. aeruginosa (20, 21). LTTRs that function in a similar fashion have not yet been identified in B. thailandensis or its pathogenic relatives.
How BGCs are silenced, and how they may be activated by using the host’s endogenous machinery, is a research area that is very much in its infancy, with important implications for bacterial physiology, microbial interactions, and drug discovery. In this study, we have examined the regulatory genes with which B. thailandensis silences its BGCs under normal growth conditions. We report the identification of ScmR (for secondary metabolite regulator), a LTTR that pleiotropically represses production of a number of BGCs in laboratory cultures, including the mal, bhc, tha, and cap gene clusters. Using global transcriptomic and metabolomic studies, we show that mutants lacking scmR are hyperactive secondary metabolite producers in vitro and in vivo. Our studies uncover a regulatory circuit, which governs virulence and secondary metabolite synthesis, and involves interplay among QS-related LuxRs and ScmR, as well as pathway-specific transcriptional regulators.
Results
Identification of scmR.
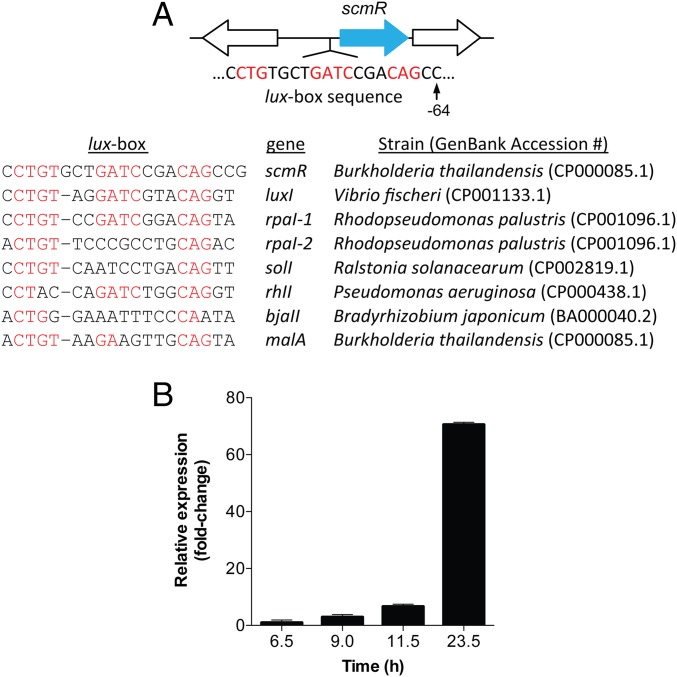
Recent RNA-seq studies by Greenberg and coworkers have allowed identification of a core set of genes that is QS-activated in the Pseudomallei group pathogens, which include B. thailandensis, B. pseudomallei, and B. mallei (19, 22). Because of the importance of LTTRs in regulating secondary metabolite production in other Proteobacteria, we searched the RNA-seq datasets for transcriptional regulators that were common to all three strains. Only one LTTR was found to be conserved among these strains and transcribed at sufficient levels in stationary phase, gene BTH_I1403 (in B. thailandensis E264) with orthologs BP1026B_I0582 and BMA2049 in B. pseudomallei Bp82 and B. mallei GB8, respectively. We refer to this gene as scmR. Its genomic context shows that it is preceded by a lux box, the sequence of which is akin to those from other Proteobacteria (Fig. 2A). It appears to be expressed from a polycistronic operon, along with its downstream gene, predicted to encode a lactate dehydrogenase.
Fig. 2.
Genetic locus of scmR. (A) ScmR is located in an operon with a putative lactate dehydrogenase (downstream); it is preceded by a lux-box, which is homologous to characterized lux-box sequences in a number of Proteobacteria, as shown in the table in A. (B) Expression of scmR in WT B. thailandensis E264 monitored by RT-qPCR. At the times shown, total RNA was isolated and reverse-transcribed to cDNA, and the levels of scmR were quantified by qPCR. Gapdh was used as an internal control at each time point. The data are normalized to the first time point at 6.5 h. Shown are averages from three biological replicates. Error bars represent SEM.
The lux box upstream of scmR indicates that it is induced as a function of QS. Indeed, RNA-seq data by the Greenberg laboratory showed an approximately twofold activation of this gene in high- vs. low-cell density cultures (19). We verified this result using quantitative RT-PCR (RT-qPCR) (Fig. 2B), which revealed accumulation of scmR transcripts as a function of cell density and up to a 70-fold induction. Furthermore, no activation of scmR occurred in a triple-btaI deletion strain, in which all three QS signal synthases have been removed (SI Appendix, Fig. S1 and Table S1) (14). Collectively, these results show that scmR is a QS-activated transcriptional regulator that is conserved in the Pseudomallei group pathogens.
Metabolomic Analysis of ΔscmR.
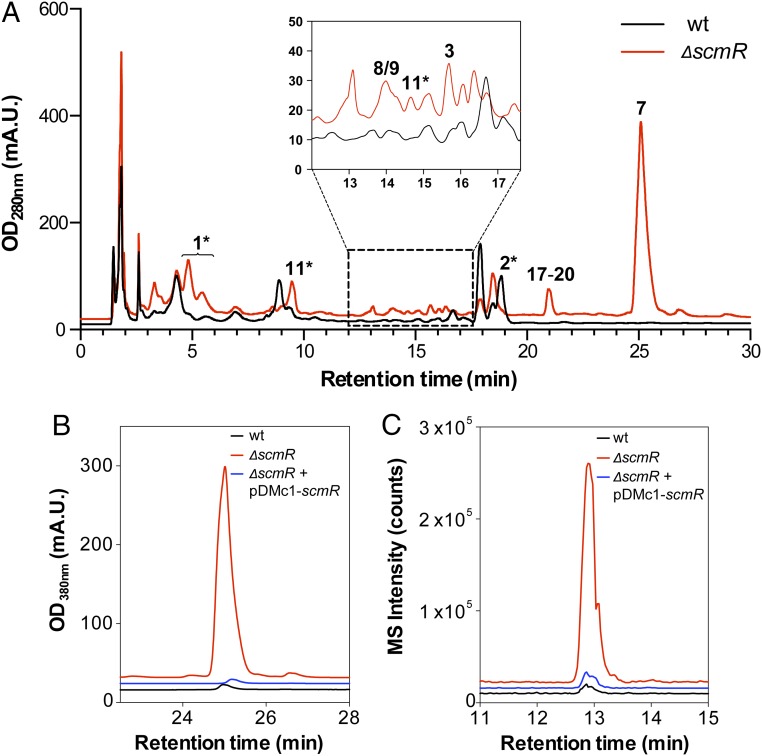
To examine a possible role for scmR in the transcriptional control of BGCs in B. thailandensis, we created an unmarked scmR deletion mutant (ΔscmR; SI Appendix, Table S1) and compared its secondary metabolic profile with that of the parental WT strain. The results showed major induction of a number of metabolites in ΔscmR (Fig. 3A)—notably, burkholdac, malleilactone, thailandamide, and acybolins (11) (23). Specifically, in the mutant strain malleilactone A, burkholdac A, thailandamide A, and acybolin A were overproduced 210-, 61-, 54-, and 18-fold, respectively. Numerous new compounds appeared to be induced as well as a result of the deletion. To verify the role of scmR in this remarkable up-regulation, complementation studies were carried out, in which a plasmid-encoded scmR was expressed in the ΔscmR mutant from a rhamnose-inducible promoter. ScmR expression led to a major down-regulation of secondary metabolite synthesis and thus rescued the mutant phenotype (SI Appendix, Fig. S2). Notably, expression of plasmid-encoded scmR returned the abundant production of malleilactone and burkholdac to WT levels. (Fig. 3 B and C). Thus, scmR greatly represses the biosynthesis of a number of known and unidentified metabolites.
Fig. 3.
Deletion of scmR leads to induction of secondary metabolism in B. thailandensis E264. (A) HPLC-MS analysis of the secondary metabolomes of WT (black trace) and ΔscmR (red trace) B. thailandensis. Peaks that can be assigned based on UV-vis or HR-MS analysis are marked (Fig. 1). The star denotes observation of multiple analogs of bactobolins, HAQs, and acybolins. (B) Complementation of the malleilactone hyperproduction phenotype in ΔscmR by expression of plasmid-encoded scmR. Levels of malleilactone production in WT (black trace), ΔscmR (red trace), and ΔscmR expressing a rhamnose-inducible, plasmid-encoded scmR (blue trace) are shown. (C) Complementation of the burkholdac hyperproduction phenotype in ΔscmR by expression of plasmid-encoded scmR. Levels of burkholdac A production in WT (black trace), ΔscmR (red trace), and ΔscmR containing a plasmid-encoded scmR (blue trace) are shown.
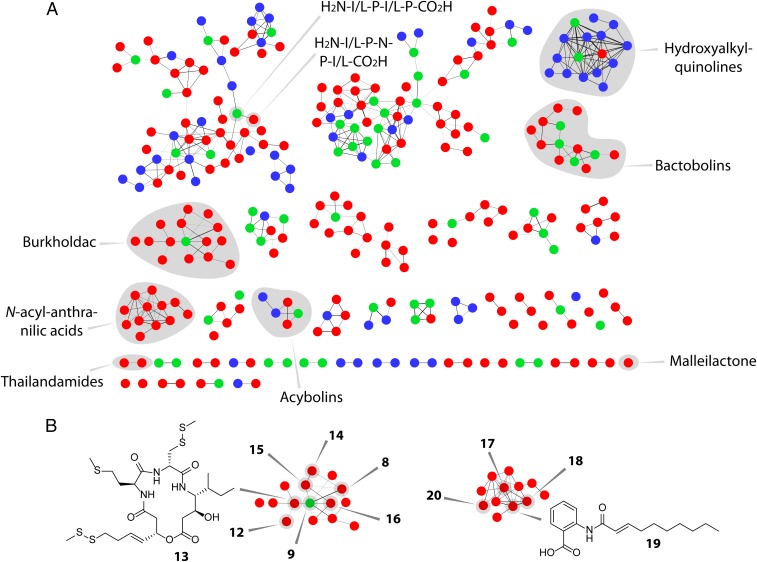
We wished to gain further insights into the scmR-regulated metabolome of B. thailandensis. To do so, we generated a mass spectral network of the secondary metabolomes of the WT and ΔscmR strains. In this method, recently pioneered by Dorrestein and coworkers, untargeted MS/MS data are used to generate a global network of all secreted small molecules (24). Each node within the network represents a distinct metabolite, and the lines connecting them are indicators of structural similarity. In the MS/MS network shown, the red, blue, and green nodes correspond to molecules detected only in ΔscmR cultures, only in WT cultures, or under both conditions, respectively (Fig. 4A). The network revealed 13 molecular families that are only observed in the scmR deletion mutant. Four families are unique to the WT, and the remainder contain molecules that are detected under both growth conditions. This initial analysis is consistent with an important role for scmR in the regulation of BGCs in B. thailandensis. It further suggests that some BGCs are repressed by scmR (red compound clusters, Fig. 4A), whereas others are activated (blue clusters).
Fig. 4.
MS/MS network analysis of the secondary metabolomes of WT and ΔscmR B. thailandensis E264. (A) Each node corresponds to a unique secondary metabolite. The thickness of a line connecting two nodes is an indicator of structural similarity. Compounds observed only in ΔscmR (red nodes), only in WT (blue nodes), or in both cultures (green nodes) are shown. Molecular families that were identified are shaded in gray and labeled. A large molecular family (top left) appears to consist of peptide fragments; the sequences of two such peptides were identified by tandem HR-MS. The acybolin family contains two previously identified variants (red nodes); other variants within this family were not detected before and have not yet been verified as acybolin relatives. (B) Compounds produced in ΔscmR. Several burkholdac analogs were identified in ΔscmR. The variants identified are marked in the burkholdac molecular cluster (8, 9, and 12–16). Similarly, structures of N-acyl-anthranilic acids are indicated in the corresponding molecular family (17–20; Fig. 1).
Using the available high-resolution (HR) MS and HR-MS/MS data, we were able to assign compounds to several molecular families (shaded in gray, Fig. 4A), including burkholdac, malleilactone, 4-hydroxy-2-alkylquinolines (HAQs), thailandamide, bactobolin, and acybolin (SI Appendix, Table S2). We were intrigued by the large molecular family of burkholdac, which appeared to contain numerous high-molecular-weight analogs. Several of these were isolated and structurally elucidated using NMR and HR-MS, leading to burkholdac C (12) and D (13), which are thiomethyl-modified derivatives of burkholdac A (8) and B (9), respectively (Fig. 4B and SI Appendix, Figs. S3–S10 and Tables S2 and S3). We also identified bis-burkholdacs A, B, and C, which were assigned by HR-MS, HR-MS/MS, and chemical degradation analysis (SI Appendix, Figs. S11 and S12 and Table S2). These derivatives consist of two burkholdac monomers—burkholdacs A-A (14), A-B (15), or B-B (16)—linked through two intermolecular disulfide bonds (Fig. 1). Reduction of the disulfide bonds with a thiol-containing reducing agent gave the anticipated monomeric products, as verified by HR-HPLC-MS, consistent with their structural assignments (SI Appendix, Fig. S11). Note that the exact position of disulfide bond formation was not identified in our studies, although the degradation analysis of 16 suggests that both combinations of intermolecular disulfide bonds are formed (SI Appendix, Fig. S12).
Aside from identifying new analogs of known compounds, the differential metabolomic analysis also provided an opportunity to discover new metabolites in the ΔscmR strain. Specifically, we found a family of aromatic compounds that were produced only in the ΔscmR strain (Fig. 4B). Isolation of four variants followed by structural elucidation via NMR yielded the N-acylated anthranilic acids shown (17–20; Fig. 4B). These structural assignments were consistent with HR-MS and tandem HR-MS studies (SI Appendix, Figs. S13–S18 and Tables S2 and S4). N-acylated anthranilic acids are previously undescribed biological molecules. Derivative 17 has been prepared synthetically and assessed for its bioactivity (25). These studies revealed significant PCAF lysine acetyltransferase inhibition, a transcriptional activator associated with p53. They also showed anticancer activities for compound 17, with IC50 values between 31 and 87 μM against eight cancer cell lines. The bioactivity of derivatives 18–20 have not yet been characterized.
Two sources can be envisioned for compounds 17–20. Anthranilic acid is a common metabolite in bacteria and a precursor for secondary metabolites, including HAQs (26, 27). It could be adorned with variable acyl groups by an acyl transferase to give metabolites 17–20. Alternatively, it has been shown that bacteria of the genus Arthrobacter express hod, a dioxygenase that can degrade the Pseudomonas quinolone signal (PQS), 2-heptyl-3-hydroxy-4(1H)-quinolone, to acylated anthranilic acids (28). Given that B. thailandensis does not encode a hod with significant homology to that of Arthrobacter, we prefer the former explanation. Nonetheless, a HAQ degradation pathway for the production of 17–20 cannot be excluded. Collectively, the data above show that scmR controls a vast secondary metabolome, repressing the production of many compounds, while activating that of others.
Transcriptomic Analysis of ΔscmR.
We next aimed to underpin the metabolomic studies above with a global transcriptomic analysis. Three biological replicates of each WT and ΔscmR were cultured to early stationary phase. Total RNA was isolated and subjected to RNA-seq. A total of 22–30 million reads were obtained per replicate, each read with an average length of 75 bp. At the chosen time point, 41% of the genome was found to be expressed in WT cells. Among the two sample sets, good reproducibility was observed with a low coefficient of variance. The data were analyzed by determining differential gene expression levels for all observed transcripts that were mapped onto the sequenced B. thailandensis E264 genome. In WT samples, the scmR mRNA was the most abundant transcriptional regulator among all of the annotated LTTRs, consistent with its important role during stationary phase.
The top-40 up-regulated genes and top-70 down-regulated genes in ΔscmR (relative to WT) are listed in Table 1 and SI Appendix, Table S5, respectively. In agreement with the metabolomic data, we observed a major induction of burkholdac, malleilactone, and bactobolin biosynthetic genes. Remarkably, the 11 genes that were up-regulated most are all part of the burkholdac BGC; they were induced 50- to 135-fold relative to WT (Table 1). The AraC-type transcriptional regulator, which has been shown to be a bhc pathway-specific positive regulator, was induced 19-fold in ΔscmR (Table 1). The remaining groups of genes from Table 1 include the malleilactone and bactobolin gene clusters, which are induced 8- to 18-fold and 7- to 13-fold, respectively. Within this list (Table 1), only eight genes are not directly involved in secondary metabolism.
Table 1.
Top-40 up-regulated genes in ΔscmR relative to WT determined by RNA-seq analysis
| Locus tag | Fold change | Gene | Joint Genome Institute product name |
| BTH_I2366 | 135.0 | bhcB | Polyketide synthase |
| BTH_I2367 | 123.7 | bhcA | Dihydroaeruginoic acid synthetase |
| BTH_I2365 | 123.1 | bhcC | Polyketide synthase |
| BTH_I2363 | 113.3 | bhcE | Polyketide synthase |
| BTH_I2362 | 98.6 | bhcF | Acyl-CoA dehydrogenase domain protein |
| BTH_I2360 | 95.7 | bhcH | Nonribosomal peptide synthetase, putative |
| BTH_I2361 | 95.2 | bhcG | Phosphotransferase enzyme family protein, putative |
| BTH_I2359 | 91.2 | bhcI | Pyridine nucleotide-disulfide oxidoreductase, class II |
| BTH_I2358 | 64.3 | bhcJ | Lipase/esterase |
| BTH_I2368 | 52.0 | bhcAa | Hypothetical protein |
| BTH_I2357 | 50.8 | bhcK | Thioesterase type II |
| BTH_I2369 | 18.8 | Transcriptional regulator, AraC family domain protein | |
| BTH_II2091 | 17.8 | malD | Adenosylmethionine-8-amino-7-oxononanoate aminotransferase, putative |
| BTH_II2089 | 16.2 | malB | Hypothetical protein |
| BTH_I3247 | 16.1 | Aldehyde dehydrogenase family protein | |
| BTH_I2356 | 15.5 | Serine protease, subtilase family | |
| BTH_II2090 | 14.9 | malC | Syringomycin synthesis regulator SyrP, putative |
| BTH_I2480 | 14.7 | Sulfate ABC transporter, periplasmic sulfate-binding protein | |
| BTH_I2450 | 13.9 | Amino acid ABC transporter substrate-binding protein, PAAT family | |
| BTH_I1849 | 13.6 | Two component transcriptional regulator, LuxR family | |
| BTH_II2093 | 13.2 | malF | Polyketide synthase, putative |
| BTH_II1234 | 12.6 | btaL | JamP |
| BTH_II2096 | 12.5 | malI | Long-chain-fatty-acid–CoA ligase, putative |
| BTH_II1236 | 12.0 | btaN | Nonribosomal peptide synthetase, putative |
| BTH_II2092 | 11.8 | malE | Aldehyde dehydrogenase family protein |
| BTH_II2088 | 11.0 | malA | Thiotemplate mechanism natural product synthetase |
| BTH_II2095 | 10.8 | malH | Diaminopimelate decarboxylase, putative |
| BTH_II2097 | 10.6 | malJ | Lipoprotein, putative |
| BTH_II1237 | 10.0 | btaO | Thiotemplate mechanism natural product synthetase |
| BTH_II2099 | 9.7 | malL | AMP-binding domain protein |
| BTH_I0892 | 9.7 | Hypothetical protein | |
| BTH_I0815 | 9.5 | Bacterial protein of unknown function (DUF934) superfamily | |
| BTH_II2098 | 9.2 | malK | Malonyl CoA-acyl carrier protein transacylase |
| BTH_I1051 | 9.0 | Sulfate transporter, putative | |
| BTH_II1240 | 8.9 | btaS | Thioesterase II of alpha/beta hydrolase superfamily |
| BTH_II1238 | 8.5 | btaP | Polyketide synthase |
| BTH_II2094 | 8.5 | malG | Ketol-acid reductoisomerase (EC 1.1.1.86) |
| BTH_II1241 | 7.8 | btaT | Drug resistance transporter, Bcr/CflA family protein, putative |
| BTH_II1239 | 7.8 | btaQ | Acetyltransferase, GNAT family |
| BTH_I0814 | 7.7 | Sulfite reductase (NADPH) beta subunit (EC 1.8.1.2) |
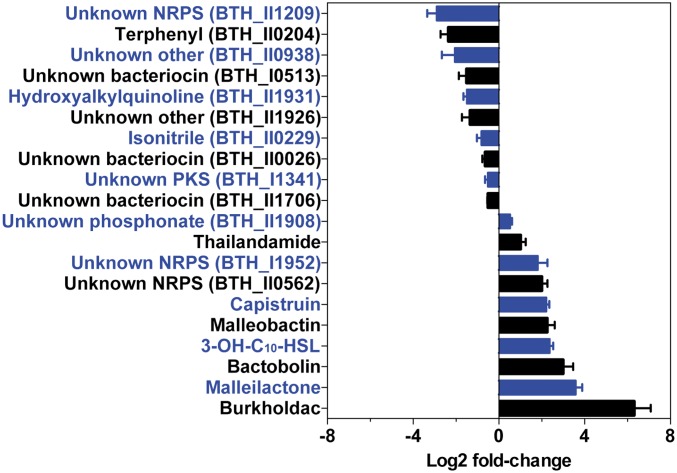
Given the significant repression of the bhc, mal, and bta BGCs by scmR, as well as the induction observed in Fig. 3A, we next examined the effect of the regulator on all BGCs in B. thailandensis (Fig. 5 and SI Appendix, Table S6). Our sequencing data captured 20 of the 23 BGCs, and of these, 13 were significantly affected by scmR, being either up- or down-regulated threefold or more. Ten were up- or down-regulated by fourfold or more (SI Appendix, Table S6). These results are in line with the metabolomic data and again underscore the importance of scmR in the regulation of secondary metabolism in B. thailandensis.
Fig. 5.
Regulation of secondary metabolism by scmR. Shown is the effect of scmR deletion on the expression of 20 secondary metabolite BGCs as determined by RNA-seq analysis. Within a BGC, the changes in expression level for each gene were averaged to give the overall fold change for the cluster, as a result of scmR deletion (see also SI Appendix, Table S6). Error bars represent SEM. The bars and corresponding labels are alternately colored for clarity.
Among the down-regulated genes, those involved in the biosynthesis of an unknown nonribosomal peptide and of terphenyl (21) were affected the most, at 4- to 14-fold and 3- to 7-fold, respectively (SI Appendix, Table S6) (29). HAQ biosynthesis was also down-regulated (approximately threefold), indicating that ScmR acts as a positive regulator for the hmq cluster, consistent with the metabolic data in the MS/MS network (Fig. 4A). Additionally, a number of stress response genes, subunits of ATP synthase, and various transcriptional regulators were also significantly affected (SI Appendix, Table S5). These results suggest that ScmR, in addition to controlling secondary metabolism, directly or indirectly activates stress response genes and ATP synthesis. One could speculate that WT cells cope with the energy demands of the stationary phase by limiting secondary metabolism and enhancing ATP synthesis, and that these are simultaneously driven by ScmR; further experiments are necessary to test this hypothesis.
Collectively, the RNA-seq analysis shows that scmR controls expression of a vast and diverse regulon. This regulon consists primarily of secondary metabolite BGCs, but also includes stress response, ATP synthase, various transcriptional regulators, and other genes, whose importance is not yet known. We found that 328 genes are either up- or down-regulated fourfold or more, and 470 are affected threefold or more, indicating that this transcriptional regulator is important to the stationary phase lifestyle of B. thailandensis.
Characterization of ΔscmR in Vivo.
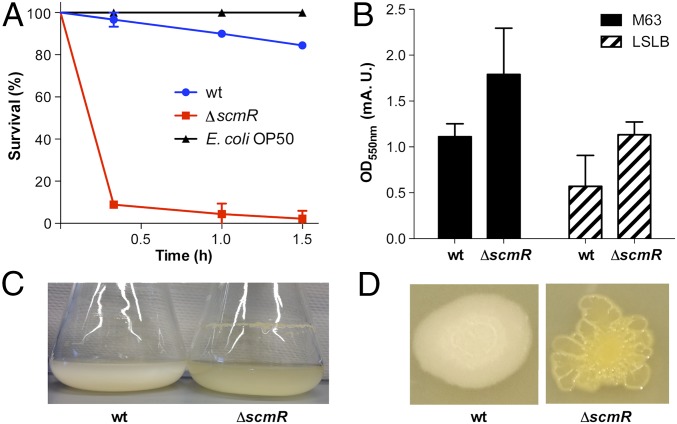
Malleilactone has been shown to be cytotoxic in a C. elegans model (15). Biosynthetic mutants that cannot generate malleilactone were found to exhibit significantly reduced virulence compared with the WT. We hypothesized that the ΔscmR strain, with its ability to overproduce malleilactone, would be hypervirulent to C. elegans. To test this hypothesis, C. elegans growth inhibition assays were carried out. WT worms were synchronized, grown to the L4 larval stage, and then transferred to an assay plate, where they were provided with either E. coli, WT B. thailandensis, or the ΔscmR mutant. Whereas WT B. thailandensis led to a slow decline in viability, the ΔscmR mutant caused remarkably rapid death. After only a 20-min incubation with ΔscmR, >90% of the C. elegans worms were killed. By contrast, >95% of the worms remained viable when treated with WT B. thailandensis for the same time span (Fig. 6A). Consistent with the transcriptomic and metabolomic studies above, secondary metabolite and virulence factor production are decidedly up-regulated in the ΔscmR strain, leading to a hypervirulent phenotype in vivo.
Fig. 6.
Hyperproduction of virulence factors by ΔscmR in vivo. (A) Survival of C. elegans when fed on E. coli (black trace), WT B. thailandensis (blue trace), or ΔscmR (red trace). Error bars represent SEM from measurement of three independent replicates (P < 0.001). (B–D) Repression of biofilm formation by scmR. (B) Crystal violet assay of WT and ΔscmR in two media, M63 and low-salt LB. Averages from three replicates are shown; error bars represent SEM (P < 0.01). (C) Flask growth of WT and ΔscmR shows enhanced biofilm formation and pigment production in the mutant relative to WT. (D) Enhanced pigmentation, corrugated colony morphology, and biofilm formation on agar plates in ΔscmR relative to WT.
Biofilm and Morphological Analysis of ΔscmR.
In Proteobacteria, induction of some secondary metabolites is linked to the onset of biofilm formation (30–33). Given that scmR primarily represses secondary metabolite production, we wondered whether it had a similar effect on biofilm formation. Indeed, the crystal violet assay showed enhanced biofilm formation in ΔscmR cells relative to the WT (Fig. 6B). This effect was corroborated by RNA-seq results, which showed induction of key biofilm formation genes in the deletion mutant (SI Appendix, Table S7) (34). Moreover, liquid cultures showed augmented pigmentation and pellicle formation in ΔscmR (Fig. 6C), and colonies grown on agar exhibited a corrugated, rugged cell morphology, in contrast to the smoother phenotype of WT B. thailandensis (Fig. 6D). Accordingly, scmR acts as a repressor of biofilm formation, virulence, and secondary metabolite production in stationary phase.
Interaction of scmR with Pathway-Specific Transcriptional Regulators.
The data above provide a snapshot of the metabolomic, transcriptomic, and morphological state of the ΔscmR mutant, and we next began to examine the regulatory mechanisms by which this state is achieved. We focused on the expression of the mal and bhc clusters, because they are most strongly repressed by ScmR (Fig. 5). In the latter case, an AraC-type regulator has been shown to act as a transcriptional activator of burkholdac production (17). In the former, we previously showed that MalR, an orphan luxR-type pathway-specific regulator, is required for induction of the mal cluster using small molecules elicitors (35, 36). To examine the interaction of scmR with these two transcriptional regulators, we created ΔmalR and ΔaraC mutants, as well as two double mutants, ΔmalRΔscmR and ΔaraCΔscmR.
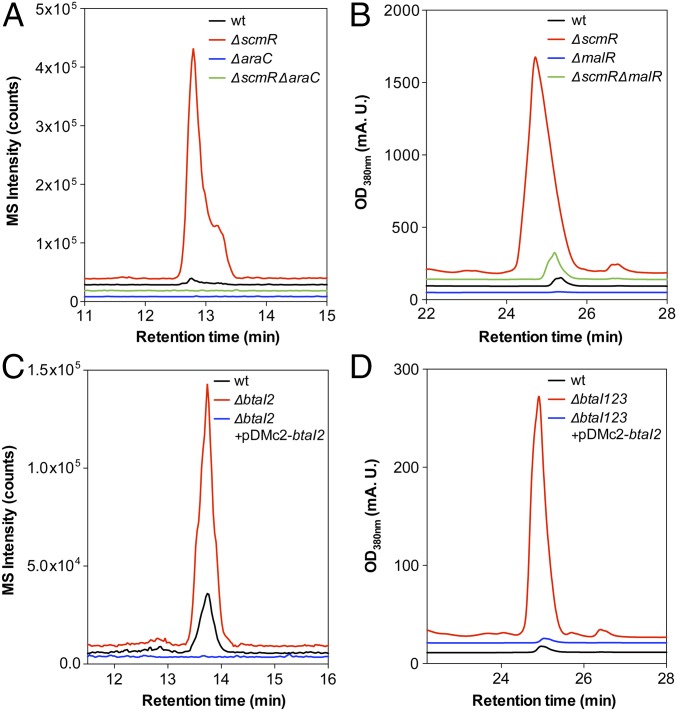
Metabolic profiles monitoring burkholdac production are shown for WT, ΔaraC, ΔscmR, and ΔaraCΔscmR strains (Fig. 7A). The WT produced minute amounts of the metabolite, whereas production was greatly enhanced in ΔscmR. The lack of any production in the ΔaraC and ΔaraCΔscmR strains indicated that the AraC-type regulator is required for induction of bhc. Thus, the scmR deletion induces the bhc cluster by acting through the AraC regulator. This conclusion is also borne out in the transcriptomic data, which show an ∼19-fold up-regulation of the araC transcript in ΔscmR relative to WT (Table 1) and suggest a model for scmR-mediated silencing of the bhc cluster (see below).
Fig. 7.
QS control of mal and bhc gene clusters via scmR and pathway-specific regulatory genes. (A) Production of burkholdac A monitored by HPLC-MS extracted ion chromatogram in WT (black trace), ΔscmR (red trace), ΔaraC (blue trace), and the double-mutant ΔscmRΔaraC (green trace). (B) Production of malleilactone monitored by HPLC-MS, measuring absorption at 380 nm (λmax of malleilactone), in WT (black trace), ΔscmR (red trace), ΔmalR (blue trace), and the double-mutant ΔscmRΔmalR (green trace). (C) Production of burkholdac A monitored by HPLC-MS extracted ion chromatogram in WT (black trace), ΔbtaI2 (red trace), and in ΔbtaI2 expressing an inducible, plasmid-encoded btaI2 (blue trace). (D) Production of malleilactone monitored by HPLC-MS, measuring absorption at 380 nm, in WT (black trace), ΔbtaI1-3 (red trace)—in which all three AHL synthases are deleted—and in ΔbtaI1-3 expressing an inducible, plasmid-encoded btaI2 (blue trace).
A similar scenario was observed with the mal cluster (Fig. 7B). Little to no malleilactone was observed in WT, in contrast to a massive overproduction with ΔscmR. The individual ΔmalR and ΔmalRΔscmR strains gave minimal amounts of malleilactone. These data again show that ScmR acts through the MalR regulator to control malleilactone production. The small amount of malleilactone observed in the double mutants suggests that other pathways, independent of malR and scmR, can lead to minor induction of the mal cluster. In our experiments, malR expression was required for activation of mal, but its expression was not affected in the ΔscmR mutant, as determined by RNA-seq. These data suggest a different model for interaction between ScmR and MalR, compared with that of ScmR with AraC (see below). They further show that ScmR can interact with two distinct pathway-specific regulators to silence the bhc and mal BGCs by probably disparate mechanisms.
Given that scmR expression is cell-density-dependent, we hypothesized that a QS-deficient mutant would exhibit enhanced secondary metabolite production in B. thailandensis. To test this idea, we compared the metabolic profile of a QS-deficient triple-btaI deletion mutant with that of WT (Fig. 7 C and D) (14). Remarkably, and consistent with our hypothesis, the triple-btaI mutant turned out to be a hyperproducer of some secondary metabolites: It exhibited a drastic 100-fold overproduction of malleilactone, 3-fold overproduction of known burkholdacs, and induction of several cryptic burkholdac variants. Expression of btaI2 from a rhamnose-inducible plasmid in the QS-deficient mutant rescued this phenotype and resulted in silencing of the bhc and mal biosynthetic pathways (Fig. 7 C and D). In fact, overexpression of btaI2 led to lower levels of burkholdac A relative to WT (Fig. 7C). Thus, QS, via ScmR, silences numerous BGCs in stationary phase and limits the secondary metabolic output of B. thailandensis.
The studies above show that the QS-deficient triple-btaI mutant and ΔscmR have similar phenotypes as determined by malleilactone and burkholdac production. To explore this relationship further, we determined the levels of AHLs using quantitative HR-HPLC-MS. We found a drastic reduction in the biosynthesis of all three AHLs, C8-HSL (115-fold), 3-OH-C8-HSL (4-fold), and 3-OH-C10-HSL (114-fold) in ΔscmR compared with the WT (SI Appendix, Fig. S19). Thus, ΔscmR behaves like a QS-deficient strain. Note that the apparent discrepancy of enhanced btaI2 transcription, yet diminished AHL production in ΔscmR, may be explained on grounds of reduced AHL precursors. AHLs are synthesized from S-adenosylmethionine, the production of which requires ATP, and fatty acid-conjugated acyl-carrier proteins. ATP levels are likely low in the ΔscmR strain (SI Appendix, Table S5). Furthermore, biofilm construction is enhanced in the mutant (SI Appendix, Table S7), and this process requires fatty acids (37, 38), which may be siphoned away from AHL synthesis, again resulting in lower levels of AHL. Together, the AHL quantification studies further underline the role of QS in silencing biosynthetic genes and curbing secondary metabolism at high cell densities.
A Model for Repression of mal and bhc by scmR.
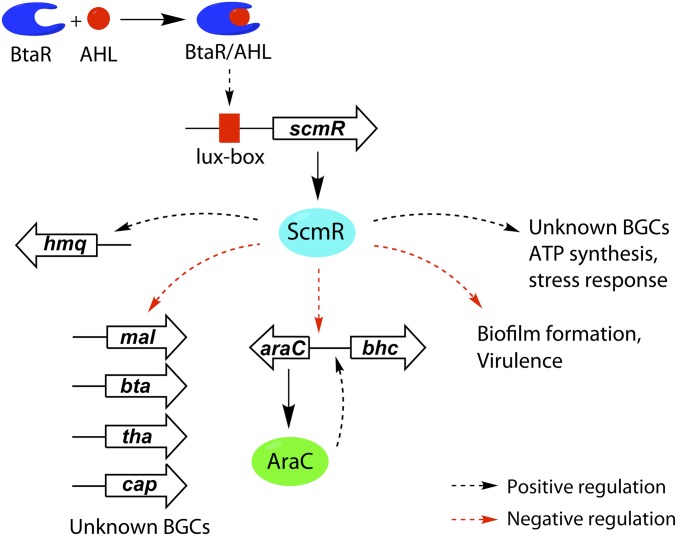
We propose the following working model, in which QS and ScmR coordinately silence mal, bhc, and other BGCs, and thus regulate secondary metabolism (Fig. 8). QS-regulated expression of scmR, via AHL signaling, leads to an abundance of ScmR in stationary phase. There, ScmR serves as a repressor of the bhc, mal, bta, cap, tha, and other gene clusters, including the AHL synthase btaI2. Note that QS- and btaI2/R2-controlled induction of scmR followed by ScmR-mediated repression of btaI2 forms a negative feedback loop, by which scmR curbs the output of the bta cluster. ScmR also serves as a repressor of araC expression, as seen in the RNA-seq experiments, and thus silences the bhc cluster. Given the down-regulation of araC transcription by ScmR, one plausible model would entail binding of ScmR to the promoter region of araC, ParaC, and thus repression of this pathway-specific regulator, which limits or eliminates burkholdac biosynthesis.
Fig. 8.
Working model for coordinated control of secondary metabolism, biofilm formation, and virulence by QS and scmR. ScmR is produced as a function of QS and accumulates in stationary phase cultures. It transcriptionally stimulates expression of the hmq cluster, as well as a number of unknown BGCs. It silences expression of the mal, bta, tha, cap, and bhc clusters, as well as genes involved in biofilm formation. Control over the mal and bhc clusters is exercised via pathway-specific regulators. In the case of bhc, expression of the araC transcriptional regulator, which is required for burkholdac production, is silenced. Red and black dotted arrows represent negative and positive ScmR-mediated transcriptional regulation, respectively.
ScmR likewise silences mal expression, but in this case, not by modulating malR transcription, because no changes in the malR transcript levels were observed in WT vs. ΔscmR cultures. At least two general models by which ScmR and MalR interact may be envisioned. One would involve competition of ScmR with MalR for the mal promoter, with ScmR binding leading to silencing of the mal cluster. This kind of competition among two transcriptional regulators for one binding site has been observed in other systems before (39, 40). Alternatively, because MalR has been shown to be an orphan LuxR with an unknown ligand (35, 41), a small molecule coinducer, only present under conditions of lowly expressed scmR (or ΔscmR), could also be invoked. Additional studies are needed to test these ideas and to provide a molecular basis for the interaction of ScmR with pathway-specific regulators of secondary metabolism.
Fig. 8 also shows additional targets of scmR-mediated control. Aside from lowering the output of the mal, bhc, bta, cap, and tha BGCs, ScmR also represses biofilm formation. Conversely, it serves as a transcriptional activator of HAQs (hmq cluster), terphenyl production, BGCs with as-of-yet unidentified products, ATP synthesis, and some stress response genes. Thus, ScmR controls a large and diverse regulon centered on virulence and secondary metabolism.
Discussion
B. thailandensis harbors an abundance of BGCs that are not expressed during normal laboratory growth. How these gene clusters are silenced has remained unknown, as has the role of QS in this process. Here, we identify and characterize a missing piece in the regulatory cascade of B. thailandensis. Genetic, transcriptomic, metabolomic, and chemical approaches show the transcriptional regulator ScmR to be an important and global gatekeeper of secondary metabolism. We demonstrate that ScmR has a significant (threefold or more) positive or negative regulatory effect on 13 of the 20 BGCs that we can monitor. Importantly, it is involved in silencing production of the known virulence factor malleilactone (15) and the HDAC inhibitor burkholdac (17), as well as in limiting production of the potent antibiotic bactobolin (10). As such, ScmR is also a key regulator and repressor of virulence factor production in B. thailandensis.
Broadly speaking, two pathways can be envisioned for activation of a silent biosynthetic pathway: one where a BGC, preceded by a weak promoter, needs to be positively regulated, or alternatively, a repressed BGC, where the repression needs to be lifted. The regulatory logic that emerges for B. thailandensis corresponds to the latter—that is, a scenario in which ScmR curbs secondary metabolism, especially the expression of mal and bhc, which would otherwise be highly expressed. Specifically, the regulatory hierarchy that emerges involves a sequence of BtaR–ScmR–AraC/MalR: BtaR promotes expression of scmR, the gene product of which interacts with pathway-specific regulators to silence or curb secondary metabolites from the mal, bhc, and bta clusters, as well as those of other BGCs (Fig. 5). Derepression via a ΔscmR mutant results in massive overproduction of virulence factors and, consequently, a hypervirulent phenotype against C. elegans. As such, scmR regulates virulence both in vitro and in vivo.
Aside from controlling secondary metabolite production, scmR also regulates other phenomena that mitigate the pressures of stationary phase growth. For example, it transcriptionally activates ATP synthesis and stress response genes, while repressing biofilm formation, possibly to facilitate the transition to a planktonic lifestyle, in which motile B. thailandensis cells can migrate to nutrient-rich environments. The expression of scmR along with lactate dehydrogenase can be rationalized on the same grounds (Fig. 2). High pH values ensue as a result of NH3 release, when B. thailandensis uses amino acids as the primary carbon source in stationary phase. Previous studies have explained the QS-controlled production of the di-acid oxalate in B. thailandensis as a strategy to maintain or control pH under this condition (42, 43). By reducing pyruvate to lactate and potentiating fermentative growth, lactate dehydrogenase can also contribute to lowering pH at high cell densities.
LTTRs that induce or repress secondary metabolite production have been found in other bacterial genera. Streptomyces coelicolor contains an abundance of LTTRs and one of these, StgR, has been shown to limit production of actinorhodin and prodigiosin in the early growth phase via interaction with pathway-specific transcriptional regulators (44). In stationary phase cultures, when biosynthesis of these metabolites is activated, stgR expression was found to be down-regulated. It will be interesting to see whether conditions that result in secondary metabolite induction in B. thailandensis (36) act through down-regulation of scmR. In general, LTTRs are better characterized in Gram-negative bacteria. As mentioned above, the best-studied example is MvfR (45–48), which regulates PQS production in P. aeruginosa and, like ScmR, is QS-activated. Unlike ScmR, however, MvfR positively regulates virulence factor production. LTTRs have a coinducer binding domain, which allows their functions to be controlled by small molecules (21). MvfR activity has been shown to be modulated by PQS (45–48). Whether ScmR is responsive to a coinducer, and, if so, the nature of such a coinducer, remains to be determined. Collectively, the examples of StgR, MvfR, and other related regulators, such as ShvR in B. cenocepacia (49), show that LTTRs can act as activators or repressors of secondary metabolite production and that diverse and complex regulatory pathways have evolved, which are mediated by LTTRs. Along with other types of transcriptional regulators of secondary metabolism, such as DasR in Streptomyces spp. (50, 51), these insights suggest that designing screens to find unknown or unsuspected regulatory proteins may provide an attractive strategy for both activating silent BGCs and, at the same time, identifying regulatory circuits that control them.
Interference with the function of MvfR results in lowered virulence by P. aeruginosa (52, 53). Consequently, MvfR has advanced to a target for the development of strategies to combat P. aeruginosa infections. Orthologs of scmR are also abundantly expressed in B. pseudomallei and B. mallei as a function of QS (22). It will be interesting to examine the roles of scmR in virulence factor production in these strains and to explore whether interfering with its function can lead to effective therapies against Pseudomallei group pathogens.
Materials and Methods
Detailed descriptions of materials and methods, including creation of mutant strains (SI Appendix, Tables S1 and S8), qPCR, RNA-seq, HPLC-MS analysis, isolation and characterization of secondary metabolites, C. elegans assays, and other procedures used are given in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Searle Scholars Program and the Princeton Intellectual Property Accelerator Fund for generous support of this work.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619529114/-/DCSupplemental.
References
- 1.Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 2.Newman DJ, Cragg GM. Natural product scaffolds as leads to drugs. Future Med Chem. 2009;1(8):1415–1427. doi: 10.4155/fmc.09.113. [DOI] [PubMed] [Google Scholar]
- 3.Walsh CT, Fischbach MA. Natural products version 2.0: Connecting genes to molecules. J Am Chem Soc. 2010;132(8):2469–2493. doi: 10.1021/ja909118a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nett M, Ikeda H, Moore BS. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat Prod Rep. 2009;26(11):1362–1384. doi: 10.1039/b817069j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X, Cheng YQ. Genome-guided discovery of diverse natural products from Burkholderia sp. J Ind Microbiol Biotechnol. 2014;41(2):275–284. doi: 10.1007/s10295-013-1376-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzel SC, Müller R. The biosynthetic potential of myxobacteria and their impact in drug discovery. Curr Opin Drug Discov Devel. 2009;12(2):220–230. [PubMed] [Google Scholar]
- 7.Brett PJ, DeShazer D, Woods DE. Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like species. Int J Syst Bacteriol. 1998;48(Pt 1):317–320. doi: 10.1099/00207713-48-1-317. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AC, Currie BJ. Melioidosis: Epidemiology, pathophysiology, and management. Clin Microbiol Rev. 2005;18(2):383–416. doi: 10.1128/CMR.18.2.383-416.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whitlock GC, Estes DM, Torres AG. Glanders: Off to the races with Burkholderia mallei. FEMS Microbiol Lett. 2007;277(2):115–122. doi: 10.1111/j.1574-6968.2007.00949.x. [DOI] [PubMed] [Google Scholar]
- 10.Seyedsayamdost MR, et al. Quorum-sensing-regulated bactobolin production by Burkholderia thailandensis E264. Org Lett. 2010;12(4):716–719. doi: 10.1021/ol902751x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duerkop BA, et al. Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensis. J Bacteriol. 2009;191(12):3909–3918. doi: 10.1128/JB.00200-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vial L, et al. Burkholderia pseudomallei, B. thailandensis, and B. ambifaria produce 4-hydroxy-2-alkylquinoline analogues with a methyl group at the 3 position that is required for quorum-sensing regulation. J Bacteriol. 2008;190(15):5339–5352. doi: 10.1128/JB.00400-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen T, et al. Exploiting the mosaic structure of trans-acyltransferase polyketide synthases for natural product discovery and pathway dissection. Nat Biotechnol. 2008;26(2):225–233. doi: 10.1038/nbt1379. [DOI] [PubMed] [Google Scholar]
- 14.Chandler JR, et al. Mutational analysis of Burkholderia thailandensis quorum sensing and self-aggregation. J Bacteriol. 2009;191(19):5901–5909. doi: 10.1128/JB.00591-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biggins JB, Ternei MA, Brady SF. Malleilactone, a polyketide synthase-derived virulence factor encoded by the cryptic secondary metabolome of Burkholderia pseudomallei group pathogens. J Am Chem Soc. 2012;134(32):13192–13195. doi: 10.1021/ja3052156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franke J, Ishida K, Hertweck C. Genomics-driven discovery of burkholderic acid, a noncanonical, cryptic polyketide from human pathogenic Burkholderia species. Angew Chem Int Ed Engl. 2012;51(46):11611–11615. doi: 10.1002/anie.201205566. [DOI] [PubMed] [Google Scholar]
- 17.Biggins JB, Gleber CD, Brady SF. Acyldepsipeptide HDAC inhibitor production induced in Burkholderia thailandensis. Org Lett. 2011;13(6):1536–1539. doi: 10.1021/ol200225v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knappe TA, et al. Isolation and structural characterization of capistruin, a lasso peptide predicted from the genome sequence of Burkholderia thailandensis E264. J Am Chem Soc. 2008;130(34):11446–11454. doi: 10.1021/ja802966g. [DOI] [PubMed] [Google Scholar]
- 19.Majerczyk C, et al. Global analysis of the Burkholderia thailandensis quorum sensing-controlled regulon. J Bacteriol. 2014;196(7):1412–1424. doi: 10.1128/JB.01405-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diggle SP, Cornelis P, Williams P, Cámara M. 4-quinolone signalling in Pseudomonas aeruginosa: Old molecules, new perspectives. Int J Med Microbiol. 2006;296(2-3):83–91. doi: 10.1016/j.ijmm.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Maddocks SE, Oyston PC. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiology. 2008;154(Pt 12):3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- 22.Majerczyk CD, et al. Cross-species comparison of the Burkholderia pseudomallei, Burkholderia thailandensis, and Burkholderia mallei quorum-sensing regulons. J Bacteriol. 2014;196(22):3862–3871. doi: 10.1128/JB.01974-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada BK, Wu Y, Mao D, Bushin LB, Seyedsayamdost MR. Mapping the trimethoprim-induced secondary metabolome of Burkholderia thailandensis. ACS Chem Biol. 2016;11(8):2124–2130. doi: 10.1021/acschembio.6b00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watrous J, et al. Mass spectral molecular networking of living microbial colonies. Proc Natl Acad Sci USA. 2012;109(26):E1743–E1752. doi: 10.1073/pnas.1203689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park WJ, Ma E. Inhibition of PCAF histone acetyltransferase and cytotoxic effect of N-acylanthranilic acids. Arch Pharm Res. 2012;35(8):1379–1386. doi: 10.1007/s12272-012-0807-2. [DOI] [PubMed] [Google Scholar]
- 26.Bredenbruch F, et al. Biosynthetic pathway of Pseudomonas aeruginosa 4-hydroxy-2-alkylquinolines. J Bacteriol. 2005;187(11):3630–3635. doi: 10.1128/JB.187.11.3630-3635.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dulcey CE, et al. The end of an old hypothesis: The pseudomonas signaling molecules 4-hydroxy-2-alkylquinolines derive from fatty acids, not 3-ketofatty acids. Chem Biol. 2013;20(12):1481–1491. doi: 10.1016/j.chembiol.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pustelny C, et al. Dioxygenase-mediated quenching of quinolone-dependent quorum sensing in Pseudomonas aeruginosa. Chem Biol. 2009;16(12):1259–1267. doi: 10.1016/j.chembiol.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Biggins JB, Liu X, Feng Z, Brady SF. Metabolites from the induced expression of cryptic single operons found in the genome of Burkholderia pseudomallei. J Am Chem Soc. 2011;133(6):1638–1641. doi: 10.1021/ja1087369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams P, Cámara M. Quorum sensing and environmental adaptation in Pseudomonas aeruginosa: A tale of regulatory networks and multifunctional signal molecules. Curr Opin Microbiol. 2009;12(2):182–191. doi: 10.1016/j.mib.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 31.López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harb Perspect Biol. 2010;2(7):a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller MB, Bassler BL. Quorum sensing in bacteria. Annu Rev Microbiol. 2001;55(1):165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 33.Fuqua C, Greenberg EP. Listening in on bacteria: Acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3(9):685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 34.Chin CY, et al. Global transcriptional analysis of Burkholderia pseudomallei high and low biofilm producers reveals insights into biofilm production and virulence. BMC Genomics. 2015;16(1):471. doi: 10.1186/s12864-015-1692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Truong TT, Seyedsayamdost M, Greenberg EP, Chandler JR. A Burkholderia thailandensis acyl-homoserine lactone-independent orphan LuxR homology that activates production of the cytotoxin malleilactone. J Bacteriol. 2015;197(21):3456–3462. doi: 10.1128/JB.00425-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seyedsayamdost MR. High-throughput platform for the discovery of elicitors of silent bacterial gene clusters. Proc Natl Acad Sci USA. 2014;111(20):7266–7271. doi: 10.1073/pnas.1400019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davey ME, Caiazza NC, O’Toole GA. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J Bacteriol. 2003;185(3):1027–1036. doi: 10.1128/JB.185.3.1027-1036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubois-Brissonnet F, Trotier E, Briandet R. The biofilm lifestyle involves an increase in bacterial membrane saturated fatty acids. Front Microbiol. 2016;7:1673. doi: 10.3389/fmicb.2016.01673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sola-Landa A, Rodríguez-García A, Amin R, Wohlleben W, Martín JF. Competition between the GlnR and PhoP regulators for the glnA and amtB promoters in Streptomyces coelicolor. Nucleic Acids Res. 2013;41(3):1767–1782. doi: 10.1093/nar/gks1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lasarre B, Aggarwal C, Federle MJ. Antagonistic Rgg regulators mediate quorum sensing via competitive DNA binding in Streptococcus pyogenes. MBio. 2013;3(6):e00333-12. doi: 10.1128/mBio.00333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patankar AV, González JE. Orphan LuxR regulators of quorum sensing. FEMS Microbiol Rev. 2009;33(4):739–756. doi: 10.1111/j.1574-6976.2009.00163.x. [DOI] [PubMed] [Google Scholar]
- 42.Goo E, et al. Bacterial quorum sensing, cooperativity, and anticipation of stationary-phase stress. Proc Natl Acad Sci USA. 2012;109(48):19775–19780. doi: 10.1073/pnas.1218092109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogul M, Carr SR. Variable ammonia production among smooth and rough strains of Pseudomonas pseudomallei: Resemblance to bacteriocin production. J Bacteriol. 1972;112(1):372–380. doi: 10.1128/jb.112.1.372-380.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao X-M, et al. Positive feedback regulation of stgR expression for secondary metabolism in Streptomyces coelicolor. J Bacteriol. 2013;195(9):2072–2078. doi: 10.1128/JB.00040-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cao H, et al. A quorum sensing-associated virulence gene of Pseudomonas aeruginosa encodes a LysR-like transcription regulator with a unique self-regulatory mechanism. Proc Natl Acad Sci USA. 2001;98(25):14613–14618. doi: 10.1073/pnas.251465298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Déziel E, et al. The contribution of MvfR to Pseudomonas aeruginosa pathogenesis and quorum sensing circuitry regulation: Multiple quorum sensing-regulated genes are modulated without affecting lasRI, rhlRI or the production of N-acyl-L-homoserine lactones. Mol Microbiol. 2005;55(4):998–1014. doi: 10.1111/j.1365-2958.2004.04448.x. [DOI] [PubMed] [Google Scholar]
- 47.Diggle SP, et al. The Pseudomonas aeruginosa quinolone signal molecule overcomes the cell density-dependency of the quorum sensing hierarchy, regulates rhl-dependent genes at the onset of stationary phase and can be produced in the absence of LasR. Mol Microbiol. 2003;50(1):29–43. doi: 10.1046/j.1365-2958.2003.03672.x. [DOI] [PubMed] [Google Scholar]
- 48.Pesci EC, et al. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1999;96(20):11229–11234. doi: 10.1073/pnas.96.20.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Subramoni S, Nguyen DT, Sokol PA. Burkholderia cenocepacia ShvR-regulated genes that influence colony morphology, biofilm formation, and virulence. Infect Immun. 2011;79(8):2984–2997. doi: 10.1128/IAI.00170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rigali S, et al. The sugar phosphotransferase system of Streptomyces coelicolor is regulated by the GntR-family regulator DasR and links N-acetylglucosamine metabolism to the control of development. Mol Microbiol. 2006;61(5):1237–1251. doi: 10.1111/j.1365-2958.2006.05319.x. [DOI] [PubMed] [Google Scholar]
- 51.Rigali S, et al. Feast or famine: The global regulator DasR links nutrient stress to antibiotic production by Streptomyces. EMBO Rep. 2008;9(7):670–675. doi: 10.1038/embor.2008.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ilangovan A, et al. Structural basis for native agonist and synthetic inhibitor recognition by the Pseudomonas aeruginosa quorum sensing regulator PqsR (MvfR) PLoS Pathog. 2013;9(7):e1003508. doi: 10.1371/journal.ppat.1003508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ji C, et al. Designed small-molecule inhibitors of the anthranilyl-CoA synthetase PqsA block quinolone biosynthesis in Pseudomonas aeruginosa. ACS Chem Biol. 2016;11(11):3061–3067. doi: 10.1021/acschembio.6b00575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.