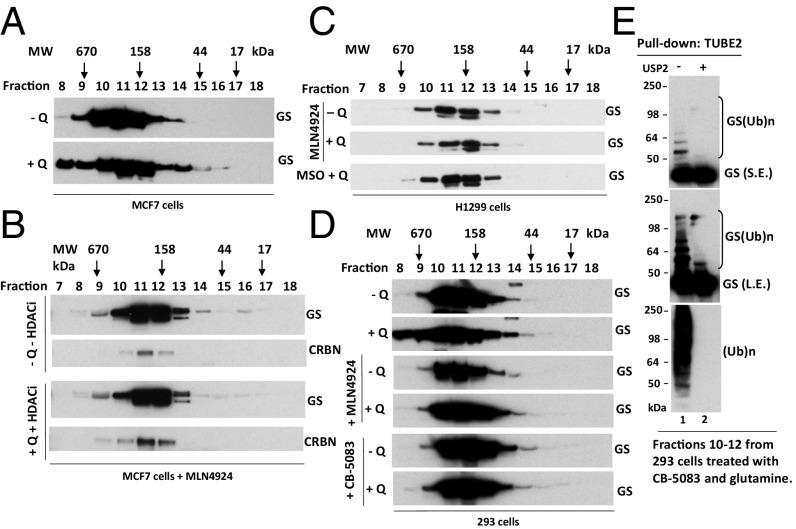
Fig. 1.
Glutamine alters the apparent assembly state of GS in an ubiquitylation- and p97-dependent manner. (A and B) MCF7 cells were starved of glutamine for 24 h and then were pretreated with (A) or without (B) 2 µM pan cullin–RING ubiquitin ligase inhibitor MLN4924 for 30 min, followed by the addition of 4 mM glutamine plus the histone deacetylase (HDAC) inhibitors suberoylanilide hydroxamic acid (SAHA) (2 µM) and NAM (10 µM) (+Q +HDACi) or not (−Q −HDACi) for 2 h. Cell lysates were fractionated on a Superdex 200 gel filtration column. Individual fractions were concentrated by trichloroacetic acid (TCA) precipitation and analyzed by SDS/PAGE and immunoblotting with the indicated antibodies. (C) As in A and B, except that H1299 cells were starved of glutamine for 24 h and then were pretreated with MLN4924 (2 μM) or the GS inhibitor MSO (2 mM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 2 h. (D) As in A and B, except that HEK293 cells were starved of glutamine for 24 h and then were pretreated with MLN4924 (2 μM) or the p97 inhibitor CB-5083 (10 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 2 h. (E) Fractions 10–12, prepared from HEK293 cells treated with CB-5083 and glutamine (used in D, bottom panel), were combined and subjected to pulldown with TUBE2 resin, followed by treatment with or without USP2. The bound fractions were analyzed by SDS/PAGE and immunoblotting with antibodies against GS and ubiquitin. L.E., long exposure; S.E., short exposure; (Ub)n, polyubiquitin.