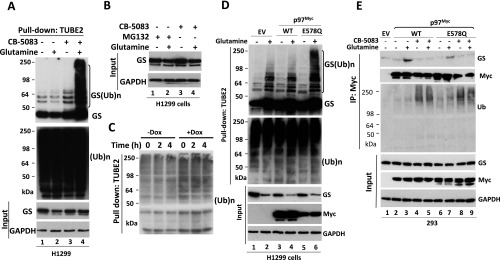
Fig. S3.
(Related to Fig. 2) p97 controls GS ubiquitylation status in response to glutamine. (A) H1299 cells were starved of glutamine for 24 h and then were pretreated with CB-5083 (10 μM) for 30 min, followed by the addition (or not) of 4 mM glutamine for 3 h. Cell lysates were fractionated on a TUBE2 resin. The bound fractions and lysate samples were analyzed by SDS/PAGE and immunoblotting with antibodies against GS, ubiquitin, and GAPDH. (Ub)n, polyubiquitin. (B) Western blot analysis showing input protein levels for Fig. 3B. (C) The bound fractions shown in Fig. 2F were analyzed by SDS/PAGE and immunoblotting with anti-ubiquitin antibody. (D) The ATP hydrolysis-deficient p97 E578Q mutant promotes the accumulation of ubiquitylated GS in response to glutamine. H1299 cells were transfected with empty plasmid (EV) or with plasmids expressing WT p97Myc or the p97Myc-E578Q mutant. Twenty-four to thirty-six hours after transfection, cells were starved of glutamine for 24 h, followed by the addition (or not) of 4 mM glutamine for 2 h. Total ubiquitinated proteins were affinity-purified using TUBE2-agarose. Bound fractions and cell lysates (input) were analyzed by SDS/PAGE and immunoblotting with the indicated antibodies. (E) The ATP hydrolysis-deficient p97 E578Q mutant binds endogenous GS. HEK293 cells were transfected with empty plasmid or with plasmids expressing WT p97Myc or the p97Myc-E578Q mutant. Twenty-four hours after transfection, cells were starved of glutamine for 24 h and were pretreated (or not) with CB-5083 (10 μM) for 30 min followed by the addition (or not) of 4 mM glutamine for 2 h. Cell extracts were immunoprecipitated with anti-Myc antibody, and the precipitated and input fractions were evaluated by SDS/PAGE and immunoblotting with the indicated antibodies.