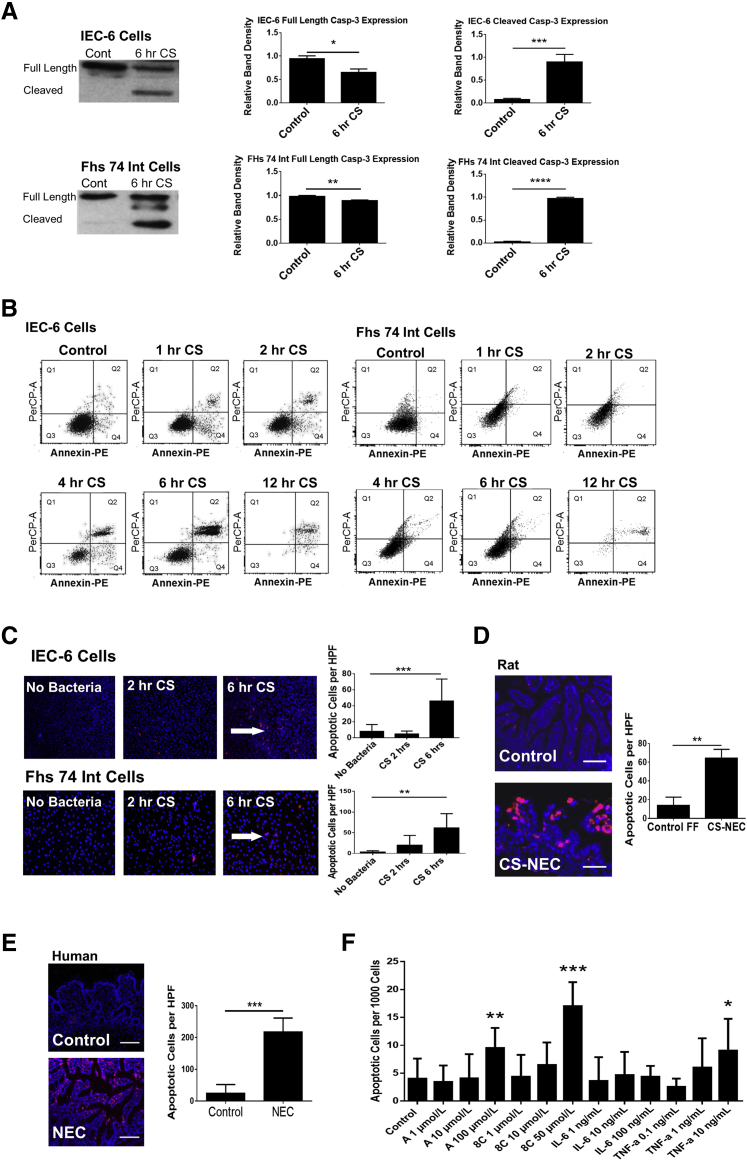
Figure 2.
A: IEC-6 and FHs 74 Int cells show a significant increase in cleaved caspase-3 after 6 hours of CS exposure. IEC-6 cells and FHs 74 Int cells were grown to near confluence in biological triplicate and treated with 107 cfu/mL of CS over a time course (0–6 hours). Western blot analysis of caspase-3 was performed to assess apoptosis. IEC-6 cells had a significant increase in cleaved caspase-3 after 6 hours of CS treatment. Full-length caspase-3 was also decreased. Similarly, FHs 74 Int cells showed a significant reduction of full-length caspase-3 and a significant increase in cleaved caspase-3 after 6 hours of CS treatment. B: Apoptosis is increased in intestinal epithelial cells infected with CS. IEC-6 and FHs 74 Int cells were grown to near confluence in triplicate and treated with 107 cfu/mL of CS over a time course (0–12 hours). The cells were washed and stained with Annexin PE to assess apoptosis with flow cytometry. Flow cytometry revealed a significant increase in early apoptosis at the 1-hour time point in both cell lines when treated with CS. C: CS induces apoptosis in IEC-6 and FHs 74 Int cells. Both IEC-6 and FHs 74 Int cells were grown in chamber slides in triplicate and were exposed to CS for 0 to 6 hours. At 6 hours, cells treated with 107 cfu/mL of CS and were found to demonstrate increased apoptosis by immunofluorescent DNA fragmentation staining (red cells indicated by white arrows). The blue stain is nuclear DAPI. The number of apoptotic cells per 1000 cells were counted and averaged from 10 random high-power views per sample. Apoptosis met clinical significance for both the IEC-6 and FHs 74 Int cells after 6 hours of infection compared with control cells not exposed to CS. D: CS-induced rat pup NEC is associated with increased intestinal apoptosis. Rat pups were born near-term and subject to either FF three times daily or dosed daily with CS (107 cfu/mL of formula) and hypoxia. Intestinal segments were cryopreserved and stained with DAPI (blue) and ApoTag for apoptosis. The FF + H + CS group had a significant increase in apoptosis compared with controls (P = 0.0025). E: Patients with NEC have increased intestinal apoptosis. Tissue samples were obtained from patients undergoing surgical bowel resection and embedded in paraffin. Slides were stained with DAPI (blue) and ApoTag for evidence of DNA fragmentation. Total apoptotic cells per high-powered field were counted. Patients with clinical NEC demonstrated increased intestinal apoptosis compared with control subjects (P = 0.0002). F: cAMP analogues induce intestinal epithelial cell apoptosis in a dose-responsive fashion. IEC-6 cells were grown to confluence of chamber-well slides and tested with various doses of PKA activators [cAMP analogues (8C and A) and cytokines (IL-6 and TNF-α)]. Slides were stained with DAPI and ApoTag to assess apoptosis. Experiments were conducted in biological triplicate. An increase in apoptosis was found with increasing concentration of PKA activators and with TNF-α [A (100 μmol/L), 8C (50 μmol/L), and TNF-α (10 ng/mL)]. IL-6 did not increase apoptosis at doses 1 ng/mL up to 100 ng/mL. Data are expressed as means ± SEM. n = 3 experiments (A and F); n = 6 per group (D); n = 4 patients with clinical NEC (E); n = 6 control subjects (E). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001. Scale bars: 50 μm (D); 200 μm (E). Original magnification, ×10 (C–E). A, adenosine 3′,5′-cyclic monophosphate; cAMP, cyclic adenosine monophosphate; Cont, control; CS, Cronobacter sakazakii; FF, formula feeding; H, hypoxia; HFs 74 Int, human small intestinal cell line; HPF, high-powered field; IEC-6, rat intestinal epithelial cell line; NEC, necrotizing enterocolitis; PE, phosphatidylethanolamine; PerCP, peridinin chlorophyll; PKA, protein kinase A; Q, quadrant; TNF-α, tumor necrosis factor α; 8C, 8-(-4-Chlorophenylthio)-2′-O-methyladenosine 3′,5′-cyclic monophosphate monosodium hydrate.