Figure 3.
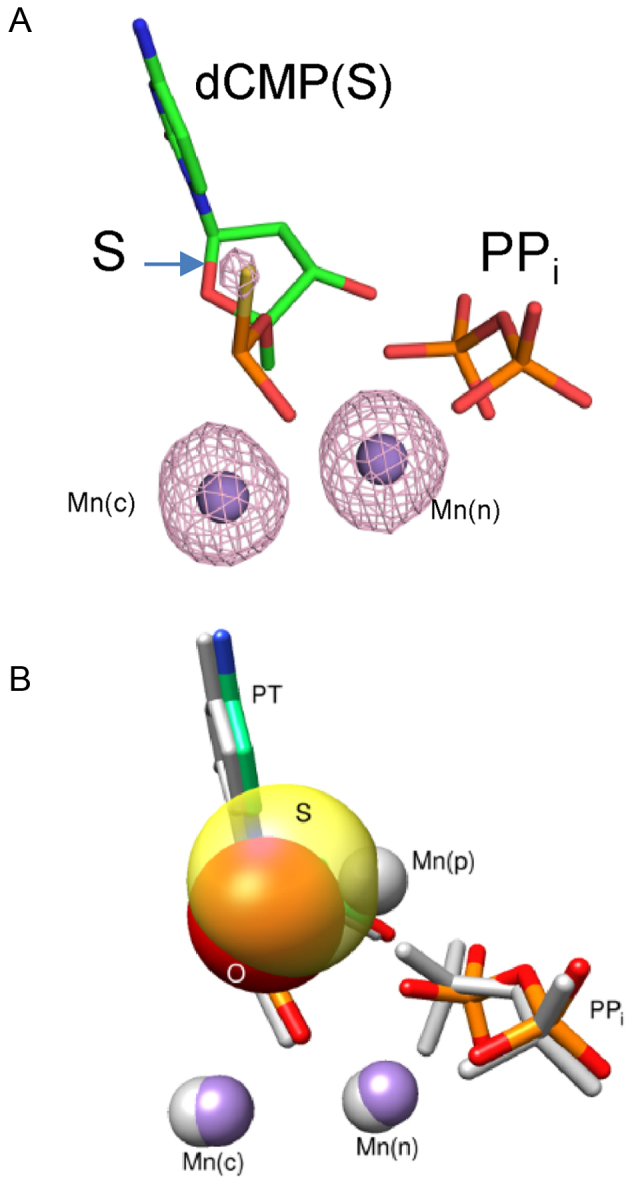
Crystallographic structure of a product complex formed after insertion of the Sp-isomer of dCTPαS. (A) Active site after insertion of the Sp-isomer of dCTPαS. An anomalous density map contoured to 5σ is shown indicating that Mn2+ occupies the catalytic and nucleotide metal binding sites. (B) Pol β active site structural comparison of the products of dCTP (PDB ID 4KLH) and Sp-dCTPαS (PDB ID 5U9H) insertion. A product metal is observed only during the incorporation of a natural nucleotide. Sulfur (transparent yellow) appears to exclude product metal binding. The equivalent oxygen of dCTP (i.e. pro-Sp) is red (appears orange in the overlap with the sulfur van der Waals radius). Three Mn2+ (gray) are observed during insertion of dCTP whereas only two manganese ions (purple) are detected during the insertion of the Sp-isomer of dCTPαS.
