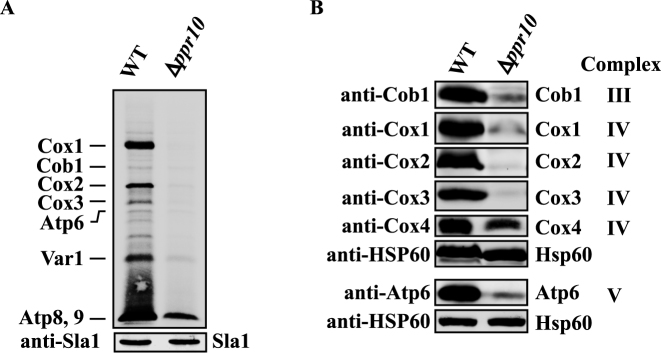
Figure 3.
Deletion of ppr10 impairs mitochondrial protein synthesis. (A) In vivo synthesis of mtDNA-encoded proteins in WT and Δppr10 cells. Mitochondrial translation products were labeled by incubating cells with [35S]-methionine/cysteine for 1.5 h in the presence of anisomycin. The labeled proteins were analyzed by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography. Sla1 detected by anti-Sla1 Ab serves as a loading control. Results presented are representative of multiple experiments. (B) Deletion of ppr10 affects the steady state levels of mtDNA-encoded proteins. Mitochondrial extracts were prepared from WT and Δppr10 cells by spheroplast lysis, and analyzed by western blotting with anti-peptide Abs against mitochondrial-encoded Cob1, Cox1, Cox2, Cox3 and Atp6, as well as nuclear-encoded Cox4. Hsp60 serves as a loading control.