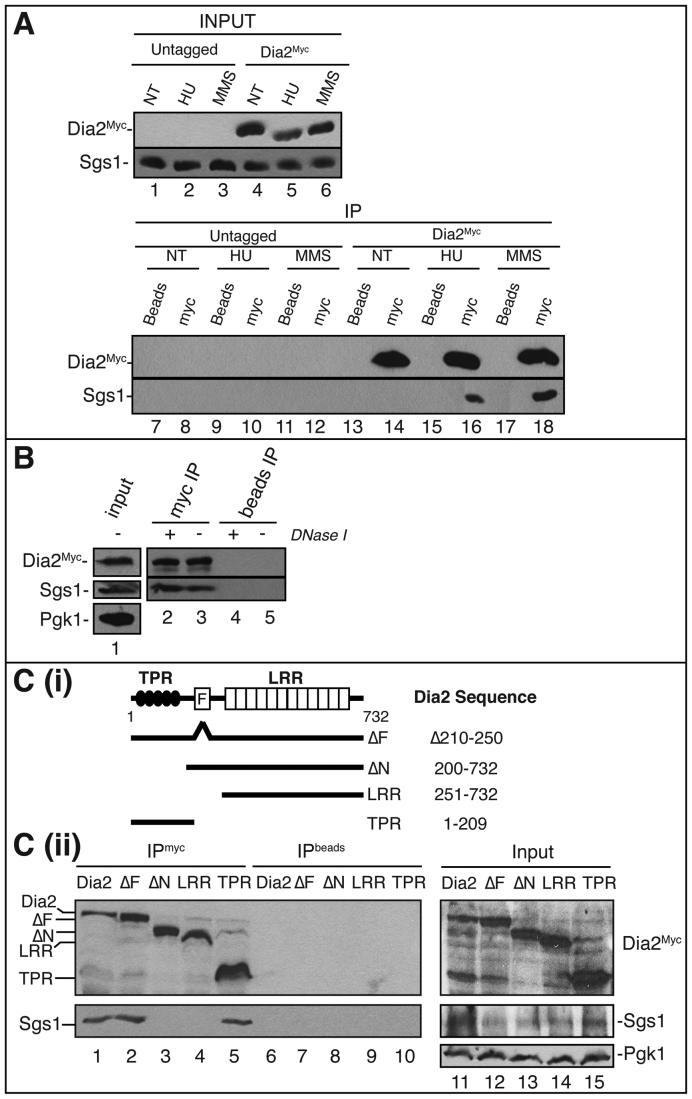
Figure 4.
Dia2-Sgs1 interaction is induced by stalled replication forks. (A) Dia2 physically interacts with Sgs1. Untreated (NT), 0.05% MMS treated or 200 mM HU treated cell lysates prepared from wild type as well as 9myc-tagged Dia2 strains were used in anti-myc immunoprecipitations (lanes 8, 10, 12, 14, 16 and 18) or incubated with protein A/G beads without antibody as a negative control (lanes 7, 9, 11, 13, 15 and 17). Immunoblots were probed with anti-myc or anti-Sgs1 antibodies. (B) Dia2 interaction with Sgs1 is not via DNA bridges. Cell lysates from wild-type cells (lane 1) were either untreated or treated with DNaseI prior to immunoprecipitation with anti-Myc antibody. (C) Sgs1 interacts with the TPR domain in Dia2. (i) Schematics showing Dia2 protein domains and mutants used in the immunoprecipitation study. TPR, tetratricopeptide repeats; F, F-Box domain; LRR, leucine-rich repeats. (ii) Strains expressing Myc-tagged Dia2 protein segments were treated with 0.05% MMS. Cell lysates (lanes 11–15) were prepared for immunoprecipitations with an anti-myc antibody (lanes 1–5) or protein A/G beads without antibody (lanes 6–10). Immunoblots were probed with anti-myc and anti-Sgs1 antibodies.