Supplemental Digital Content is Available in the Text.
Key Words: palliative medicine, HIV, Ontario, mortality, costs and cost analysis, health services
Abstract
Background:
Aging and increasing comorbidity is changing the end-of-life experience of people living with HIV (PLHIV) in the developed world. We quantified, at a population level, the receipt of health care services and associated costs across a comprehensive set of sectors among decedents with and without HIV.
Methods:
We conducted a retrospective population-level observational study of all decedents in Ontario and their receipt of health care services, captured through linked health administrative databases, between April 1, 2010 and March 31, 2013. We identified PLHIV using a validated algorithm. We described the characteristics of PLHIV and their receipt of health care services and associated costs by health care sector in the last year of life.
Results:
We observed 264,754 eligible deaths, 570 of whom had HIV. PLHIV were significantly younger than those without HIV (mean age of death 56.1 years vs. 76.6 years, [P < 0.01]). PLHIV spent a mean of 20.0 days in an acute care hospital in the last 90 days of life compared with 12.1 days for decedents without HIV (P < 0.01); after adjustment, HIV was associated with 4.5 more acute care days (P < 0.01). Mean cost of care in the last year was significantly higher among PLHIV ($80,885.62 vs. $53,869.77), mostly attributable to acute care costs.
Interpretation:
PLHIV in Ontario are dying younger, spending more time and dying more often in hospital, and incur significantly increased costs before death. Greater involvement of community-based palliative care may improve the dying experience for this complex population.
INTRODUCTION
As people with HIV age, they are becoming an increasingly medically complex population. The share of people newly diagnosed with HIV who are older than 50 is increasing, especially among men.1 Those with long-standing HIV on combination antiretroviral therapy (ART) also benefit from significant increases in longevity.2,3 With age, people living with HIV (PLHIV) are likely to acquire additional multimorbidity related to normal aging as well as from the effects of HIV and its treatment, including renal failure, hypertension, and diabetes.4–10 This demographic shift is changing the end-of-life experience of PLHIV in the developed world where mortality from non–AIDS events—including chronic diseases, substance abuse, and non-AIDS–defining cancers—now exceeds that of AIDS-defining conditions among individuals receiving ART.6,7
This rising multimorbidity and concomitant polypharmacy have led to escalating costs of care among PLHIV.11–15 In addition to the cost of ART therapy, higher costs arise among people with more advanced HIV disease, usually as a result of inpatient admissions.11–13,15–17 Regardless of disease status, health care costs among PLHIV remain significantly higher than those without HIV across all sectors of care (outpatient, inpatient, emergency department, and non–ART medication costs).11,14 At the population level, we know that costs of care at the end of life are high, are dominated by acute care, and escalate in the last 120 days of life.18 Overall, total costs of delivering care to PLHIV increase with age19 and escalate toward the end of life.12,13 However, little is known about the end-of-life care and cost across all health care sectors among PLHIV, and how they differ to those without HIV.
We had two objectives to this study: first, to describe the places of care that PLHIV experience near the end of life, including where they ultimately die, and to quantify the costs of end-of-life care among all PLHIV in Ontario, the Canadian province with the highest number of PLHIV. Ontario has a single payer health care system with universal access to most physician services, hospital admissions, emergency department visits, long-term care, home care, and continuing care/inpatient rehabilitation, as well as prescription medication costs for people older than 65 and those on social assistance.
METHODS
Data Sources
We conducted a retrospective, population-based study among PLHIV in Ontario, Canada. We used the multiple databases available from the Institute for Clinical Evaluative Sciences (ICES), a comprehensive collection of administrative claims and billing data in the province of Ontario. These deidentified databases are linked at the individual level using encrypted health care numbers as unique identifiers and are made available through a data-sharing agreement with the Ministry of Health and Long-Term Care. Ethics approval was obtained from the Sunnybrook Health Sciences Centre Research Ethics Board in Toronto, Canada and from the Ottawa Health Science Network Research Ethics Board in Ottawa, Canada.
The databases used include the Registered Persons Database, which includes demographic and mortality data for all residents eligible for provincial health care; 2006 Statistics Canada census data to link postal code of residence to attribute the household income quintile, as a proxy for socioeconomic status; the Ontario Health Insurance Program (OHIP) billing claims system, which contains 95% of physician and laboratory services conducted in the province; the Discharge Abstract Database which captures all provincial hospital admission discharge data; the National Ambulatory Care Reporting System, which contains information on emergency department and selected outpatient visits; Immigration, Refugees, and Citizenship Canada data, which contain information on individuals granted permanent residency in Canada; the Continuity Care Reporting System, which contains resident information for all publicly funded residential care homes with 24-hour nursing care (ie, nursing homes) and those in designated complex, nonacute institutional care beds; the National Rehabilitation Reporting System, which provides data on adult inpatient rehabilitation care; the Home Care Database, which collects service data on home care services, the Client Agency Program Enrolment registry, which tracks patient enrolment to individual family physicians; and the Ontario Drug Benefits, a claims database of publicly funded prescriptions for individuals aged 65 and older and those receiving social assistance (Ontario Works, Ontario Disability Support Program), or eligible for the subsidized catastrophic access Trillium program.
Study Population
We used the Registered Persons Database to capture all deaths in the province over the 3-year period from April 1, 2010 to March 31, 2013. Our study population included decedents eligible for provincial health insurance. We identified PLHIV on April 1, 2010, using an algorithm previously validated in Ontario—based on physician claims with a diagnosis of HIV—with a sensitivity of 96.2% (95% confidence interval: 95.2% to 97.9%) and specificity of 99.6% (95% confidence interval: 99.1% to 99.8%).20 Decedents not meeting this HIV ascertainment algorithm comprised the comparison group.
Variable Definition
We used neighborhood-level postal codes linked to 2006 Statistics Canada census data to assign income quintiles and rurality scores at one year before death.21 We used data from the Immigration, Refugees, and Citizenship Canada database to categorize patient immigration status as follows: Canadian born or long-standing immigrant (as immigration data are only available after 1985), immigrant from an HIV-endemic country, or immigrant from an HIV nonendemic country. The Johns Hopkins Adjusted Clinical Group System was used to ascertain multimorbidity by assigning patients to up to 32 distinct Aggregated Diagnosis Groups (ADGs), weighting these ADGs and creating a composite measure, the ADG score, which has been previously shown to be highly predictive of one-year mortality.22–24 We used the Ontario Drug Benefits claims database to identify individuals who were eligible for public drug coverage and those who had actual claims during the study period, including specifically for any ART during the study period. Home care services were ascertained as palliative or nonpalliative based on the Service Recipient Code, an administrative flag which determines eligibility for services, captured in the Home Care Database. We used a previously validated algorithm looking at the percentage of claims designated as palliative care to identify whether home visits were performed by a designated specialist in palliative care.25 Finally, we used the provincial Client Agency Program Enrolment (CAPE) registry to identify family physician remuneration models. Fee-for-service models primarily reimburse physicians based on a per-visit fee schedule. Capitation models are those that primarily reimburse physicians based on age- and sex-based capitation rates for rostered patients, with some additional funding for allied health support.
Outcomes
Health Care Cost
We report costs by sector in the last year of life. We have adopted a payer Ministry of Health and Long-Term Care (MOHLTC) costing perspective, using person-level health care expenditures that accounts for data for health care utilization and cost information per use. We used previously described methods to assign the costs of all health care records paid for by the MOHLTC.26 Briefly, cost information for sectors (eg, hospital admissions, complex continuing care, and inpatient rehabilitation) that have global budgets (eg, by institution or by health region) is determined using a top–down approach through case-mix methodology. Sectors that have fee payments associated with each use (eg, drug costs or physician fees) have costs estimated directly. All costs were expressed in 2013 Canadian dollars; we inflated past costs using health care–specific yearly consumer index reported by Statistics Canada. Health sector cost for the population was the sum of all costs among decedents captured within each respective sector.
Receipt of Services
We report receipt of care by sector in the last 90 days of life. We categorized places with receipt of care as the number of days spent in the following care settings: hospital (includes acute care, complex continuing care, designated inpatient rehabilitation beds, and emergency departments) and at home with receipt of home care. Days spent in more than one setting were counted for each of the places with receipt of care. We reported location of death in hospital or in the community. We defined palliative hospitalizations as any hospital admission during which receipt of palliative care was reported as a contributing reason for admission, or if care was provided by a palliative care team.27 We defined receipt of home care services as those provided by publicly funded Community Care Access Centres and, when provided, we categorized these as with receipt of palliative care if an end-of-life designation was given.27 We determined whether decedents had received one or more physician home visits and categorized these as having palliative specialist involvement if they had received one or more home visits from a palliative specialist.27
Analysis
We used frequencies and proportions to report characteristics of the study populations. All statistical tests were 2-tailed, and P = 0.05 was used to determine statistical significance. We used multivariable linear regression analysis to determine the association between decedent characteristics and the number of days spent in hospital in the last 90 days of life, adjusted for decedent HIV status, age, sex, neighborhood income quintile, neighborhood rurality, ADG score, immigrant status, primary care model, whether the decedent received home care services in the last year of life, whether the decedent had received palliative, nonpalliative, or no physician home visits in the last 90 days of life, and any receipt of ART in the last year of life. We used SAS 9.3 (SAS Institute Inc., Cary, NC) for all analyses.
RESULTS
Study Population Characteristics
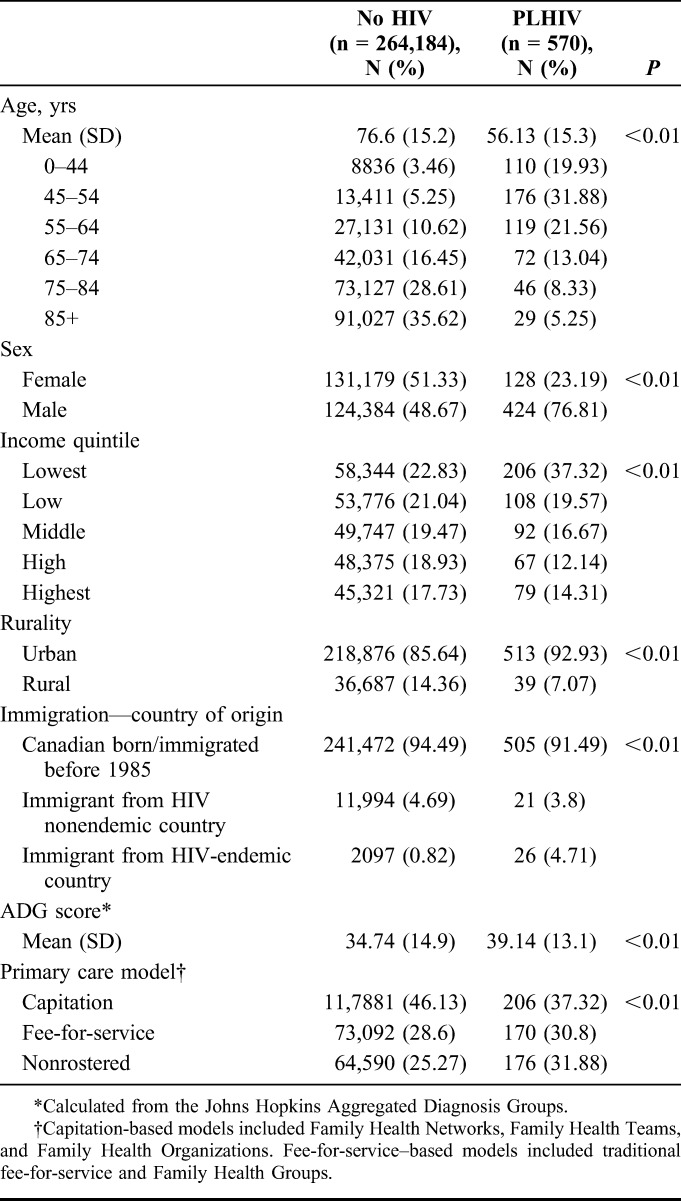
We observed 264,754 eligible deaths during our 3-year study period: 570 among PLHIV and 264,184 among people without HIV (Table 1). PLHIV were significantly younger than those without HIV: mean age of death was 56.1 years (SD 15.3 years) among PLHIV vs. 76.6 years (SD 15.2 years) among those without HIV (P < 0.01). PLHIV were more likely to be male, to live in the lowest income neighborhoods in urban settings, and to have emigrated from an HIV-endemic country. PLHIV also had higher ADG score than those without HIV and were more likely to receive care in fee-for-service models of primary care vs. newer capitation-based models.
TABLE 1.
Characteristics of PLHIV and Decedents Without HIV, Ontario, Canada, 2010–2013
Health care utilization at the end of life differed significantly between PLHIV and those without HIV (Table 2). PLHIV spent significantly more days in receipt of acute care in the last 90 days of life than decedents without HIV (20.0 vs. 12.1 days, P < 0.01) and were more likely to die in hospital [361 (65.4%) vs. 137,083 (53.6%), P < 0.01]. Of note, only 29 PLHIV (5.3%) died in a long-term care facility compared with 44,619 (17.5%) of decedents without HIV (data not shown).
TABLE 2.
Location of Death and Places of Care in Last 90 Days of Life by HIV Status
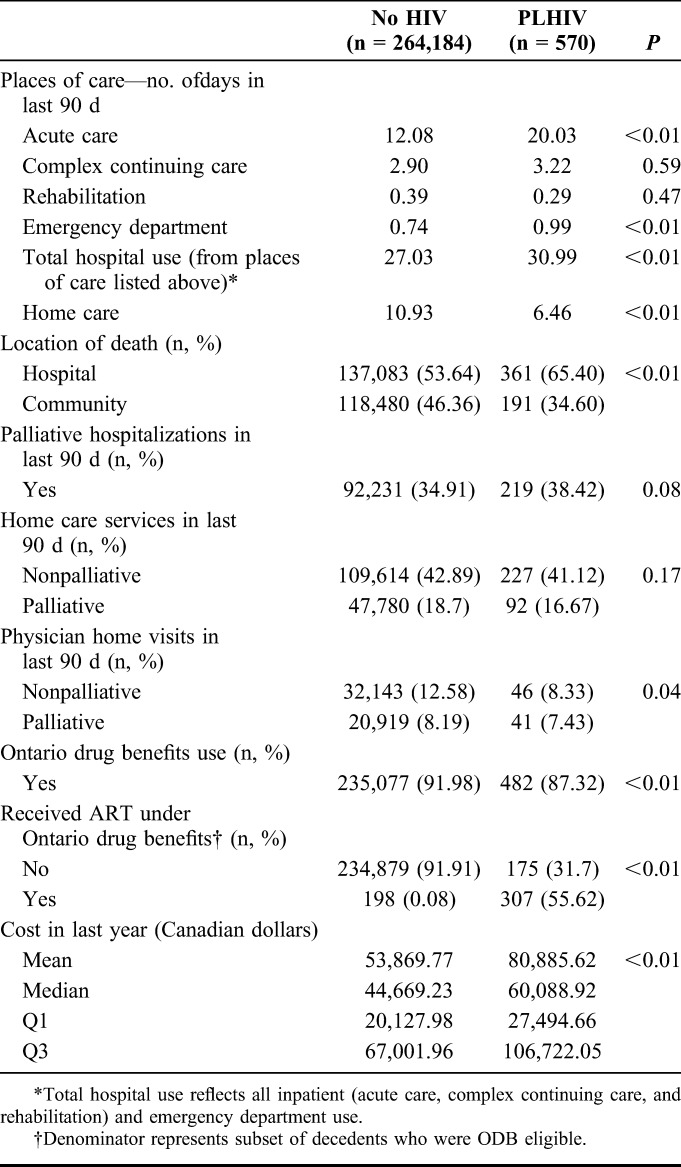
Receipt of palliative care services also differed between cohorts. PLHIV had greater hospitalizations designated as for receipt of palliative care than those without HIV [219 (38.4%) vs. 92,231 (34.9%)]. Although there were no differences in receipt of palliative home care between the cohorts, PLHIV were less likely to receive a physician visit them at home [87 (15.8%) vs. 53,062 (20.8%)]. Overall, mean costs of care in the last year of life among PLHIV were significantly higher than those without HIV ($80,885.62 vs. $53,869.77).
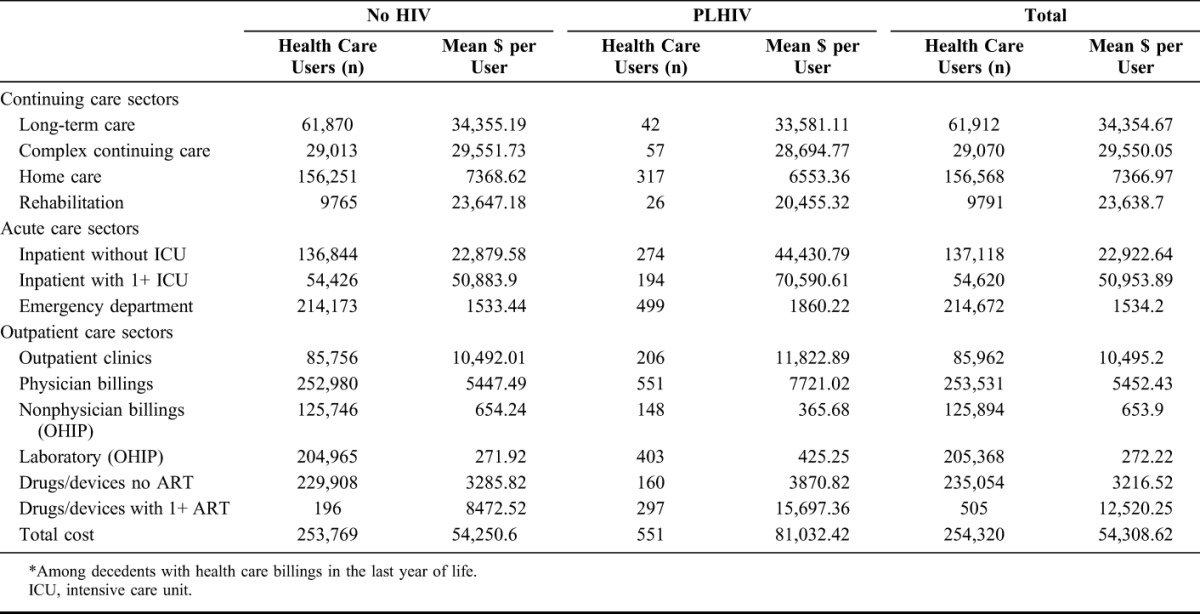
Table 3 delineates the mean cost of care per decedent for individual sectors of the health care system. The key drivers of higher costs among PLHIV are borne in inpatient care, with mean costs per user almost double than for decedents without HIV (mean cost per person for nonintensive care stay $44,430.79 vs. $22.879.58).
TABLE 3.
Mean Costs (Canadian Dollars) per Decedent in the Last Year of Life* by Sector, Ontario, Canada, 2010–2013
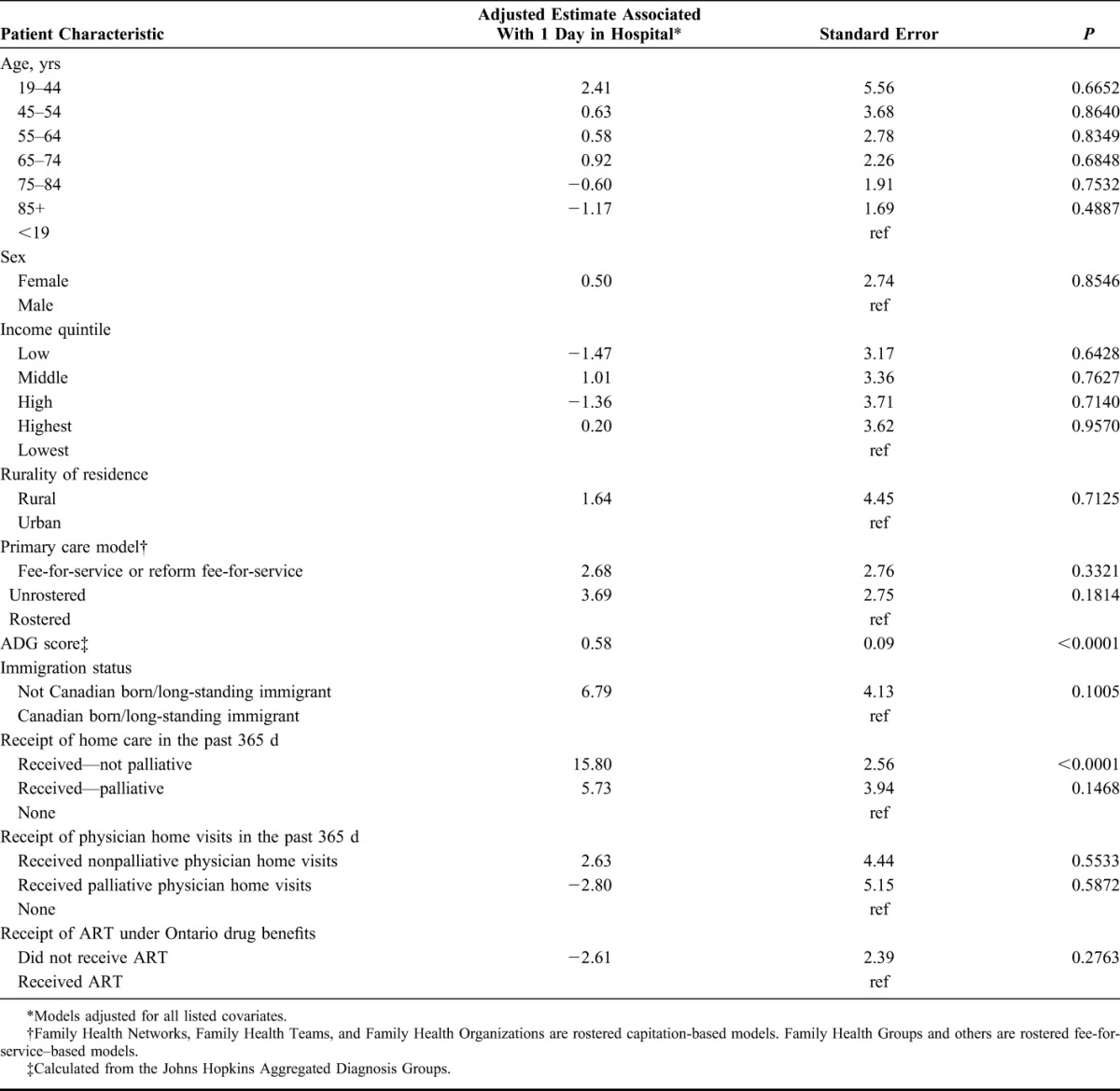
Among all decedents, after adjustment, PLHIV were significantly associated with the number of days spent in hospital in the last 90 days of life [estimate 4.48, P < 0.0001 (Supplemental Digital Content, Table 1, http://links.lww.com/QAI/A960)]. Table 4 shows the results of our multivariable linear regression model estimates of the association between patient characteristics and number of days in hospital in the last 90 days of life among restricted to PLHIV. Among PLHIV (Table 3), after adjustment, only higher ADG score (estimate 0.60, P < 0.0001), receiving nonpalliative home visits (estimate 16.91, P < 0.0001), and not receiving any Ontario Drug Benefit prescriptions (ODB) (estimate 12.82, P = 0.002) were statistically associated with having spent more days in hospital in the last 90 days of life. Parameter estimates of receiving palliative home care (c.f., those receiving home care) and being rostered to a family physician in a capitation model suggested reduction in days spent in hospital, although the large standard errors led to nonsignificant P-values.
TABLE 4.
Multivariable Linear Regression Model of the Association Between Patient Characteristics and Number of Days Spent in Hospital in Last 90 Days of Life Among PLHIV (n = 570)
INTERPRETATION
In this population-based study, we found important differences in the end-of-life experience among PLHIV and without HIV. In particular, despite advances in and access to ART at the time of our study, PLHIV were significantly younger than those without HIV, with a mean age of death of about 20 years younger. Their care at the end of life was dominated by receipt of acute care, with PLHIV spending on average 8 additional days in receipt of acute care in the last 90 days of life and more likely to die in hospital settings. This increased receipt of acute care use led to substantial costs of care in the last year of life, with health care costs for PLHIV 50% greater, or more than $27,000 more per person, than those without HIV. These findings have important implications as the population of PLHIV becomes older and increasingly complex, and presents an opportunity to improve both population costs and patient-oriented quality of end-of-life care for this population. We are the first to show end-of-life health care use and cost of a population-based HIV cohort across a wide array of health sectors.
ART has led to substantial increases in longevity among PLHIV in developed settings; today, a 20-year-old adult on ART has a life expectancy approaching that of the general population.2 However, our study reinforces that PLHIV are still dying much earlier than their non-HIV counterparts, and remain more marginalized with lower income. Such marginalization among people nearing the end of life is consistent with findings of the long-term care and home care population in Ontario in which PLHIV represent a minority population burdened by psychosocial and medical complexity.28 There is substantial evidence that poor control of HIV contributes to higher costs, mostly due to the receipt of acute care,11–17 and that receipt of ART is associated with higher medication costs but lower overall acute care costs.15 We did not find ART use to be associated with fewer acute care days; however, this finding may be confounded by the likelihood that drug costs are institutionally covered while patients are in receipt of acute care, thus may not appear within ODB claims.
The disproportionate acute care use, and resultant increased proportion of PLHIV dying in hospital settings, also has important patient-level implications. Most Canadians would prefer to die at home.29–31 However, PLHIV were less likely to receive palliative home visits near the end of life, consistent with low use in previous studies32 and despite evidence that receipt of palliative care in the home is associated with increased likelihood of dying at home.33 Furthermore, few PLHIV died in long-term care facilities; this is partially a reflection of younger age at death, and highlights the need to understand, develop, and implement patient-centered care settings that address the complex needs of an aging HIV population.34–37 In particular, aging PLHIV require a focus on mental health,28 chronic pain management,28 and polypharmacy,28 as well as mechanisms to address social needs including isolation and stigmatization,28,37 economic hardship,37,38 and housing stability.39 These challenges are in stark contrast to those faced from the typical nursing home population, typically older than 80 years with cognitive and physical disability resulting from dementia and senescence.40 Given the diversity of the population of PLHIV, these needs may be heterogeneous in nature41 and may differ from the priorities of their health care providers.39,42
Our data sets allow us to quantify care among a geographically and ethnically diverse population of PLHIV, reducing biases inherent in clinical cohort data. Furthermore, we were able to quantify comprehensive use and costs of care across an array of health care sectors. However, there are limitations to our findings. First, we were only able to identify people who were diagnosed with HIV by the health care system. We did not capture those who did not know they have HIV, and those who did but chose not to seek treatment. Misclassification of these individuals would attenuate any differences we see between the population with and without HIV. Second, although we were able to capture most costs within our single payer system, we were unable to ascertain direct costs paid out of pocket or through personal insurance, including private care, allied health support, and medications (including costly ART) paid for outside the Ontario Drug Benefit program (13% of PLHIV have no coverage). We were also unable to determine the indirect costs of care, such as that to informal caregivers, which can be substantial at the end of life.43 For the same reasons, our results may not be generalizable to other nonuniversal health care systems. Third, there is substantial evidence that poor control of HIV contributes to higher costs, mostly due to receipt of acute care11–17: as we could not ascertain immune status from our data, nor the age at or time from HIV diagnosis, HIV disease stage may play an unmeasured factor in our findings. Finally, there are likely unmeasured health and social determinants that influence access to care and services that are unmeasured in this study.44
Our study highlights that even in a system with fairly comprehensive health and social supports, PLHIV face considerably poorer health outcomes at the end of life despite higher costs of care. In perspective, the mean cost in the last year of life for PLHIV ($81,032.42) was substantially higher than those with cancer ($54,457) or congestive heart failure ($59,165) (P. Tanuseputro, June 14, 2016). These findings present a challenge to health care planners in determining the most effective and cost-efficient ways to provide end-of-life care to complex patients, including the integration of patient-centered HIV services37 with earlier community-based palliative care services.45 This study provides a signal of suboptimal end-of-life services among PLHIV; future work should explore the determinants of this experience and identify opportunities to optimize the quality and cost effectiveness of care, in particular the receipt of appropriate palliative care services.
Footnotes
Supported by a research grant from the Ontario MOHLTC to the Health System Performance Research Network (HSPRN #06034). Also supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). C.E.K. is supported by a Canadian Institutes of Health Research—Ontario HIV Treatment Network New Investigator Award.
The authors have no conflicts of interest to disclose.
The opinions, results, and conclusions reported in this article are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred. Parts of this material are based on data and information compiled and provided by Canadian Institute for Health Information (CIHI). However, the analyses, conclusions, opinions, and statements expressed herein are those of the author, and not necessarily those of CIHI.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jaids.com).
REFERENCES
- 1.Public Health Agency of Canada. HIV and AIDS in Canada: Surveillance Report to December 31, 2013. Ottawa, Ontario, Canada: Public Health Agency of Canada; 2014. [Google Scholar]
- 2.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson S, Cescon A, Samji H, et al. Life expectancy of HIV-positive individuals on combination antiretroviral therapy in Canada. BMC Infect Dis. 2015;15:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasse B, Ledergerber B, Furrer H, et al. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis. 2011;53:1130–1139. [DOI] [PubMed] [Google Scholar]
- 5.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53:1120–1126. [DOI] [PubMed] [Google Scholar]
- 6.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360:1815–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sackoff JE, Hanna DB, Pfeiffer MR, et al. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145:397–406. [DOI] [PubMed] [Google Scholar]
- 8.Goulet JL, Fultz SL, Rimland D, et al. Aging and infectious diseases: do patterns of comorbidity vary by HIV status, age, and HIV severity? Clin Infect Dis. 2007;45:1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendall C, Wong J, Taljaard M, et al. A cross-sectional, population-based study measuring comorbidity among people living with HIV in Ontario. BMC Public Health. 2014;14:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vance DE, Mugavero M, Willig J, et al. Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care. 2011;22:17–25. [DOI] [PubMed] [Google Scholar]
- 11.Guaraldi G, Zona S, Menozzi M, et al. Cost of noninfectious comorbidities in patients with HIV. Clinicoecon Outcomes Res. 2013;5:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gebo KA, Fleishman JA, Conviser R, et al. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010;24:2705–2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nosyk B, Lima V, Colley G, et al. Costs of health resource utilization among HIV-positive individuals in British Columbia, Canada: results from a population-level study. Pharmacoeconomics. 2015;33:243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Haider S, Nedrow K, et al. HIV economic burden of illness in the Veterans Health Administration population. AIDS Care. 2015;27:123–131. [DOI] [PubMed] [Google Scholar]
- 15.Barnett PG, Chow A, Joyce VR, et al. Determinants of the cost of health services used by veterans with HIV. Med Care. 2011;49:848–856. [DOI] [PubMed] [Google Scholar]
- 16.Levy A, Johnston K, Annemans L, et al. The impact of disease stage on direct medical costs of HIV management: a review of the international literature. Pharmacoeconomics. 2010;28(suppl 1):35–47. [DOI] [PubMed] [Google Scholar]
- 17.Solem CT, Snedecor SJ, Khachatryan A, et al. Cost of treatment in a US commercially insured, HIV-1-infected population. PLoS One. 2014;9:e98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanuseputro P, Wodchis WP, Fowler R, et al. The health care cost of dying: a population-based retrospective cohort study of the last year of life in Ontario, Canada. PLoS One. 2015;10:e0121759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krentz HB, Gill MJ. Increased costs of HIV care associated with aging in an HIV-infected population. HIV Med. 2015;16:38–47. [DOI] [PubMed] [Google Scholar]
- 20.Antoniou T, Zagorski B, Loutfy MR, et al. Validation of case-finding algorithms derived from administrative data for identifying adults living with human immunodeficiency virus infection. PLoS One. 2011;6:e21748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kralj B. Measuring “rurality” for purposes of health care planning: an empirical measure for Ontario. Ont Med Rev. 2000;67:33–52. [Google Scholar]
- 22.Huntley AL, Johnson R, Purdy S, et al. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. Ann Fam Med. 2012;10:134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.The Johns Hopkins Adjusted Clinical Groups (ACG) System. Baltimore, Maryland: Johns Hopkins University Press; 1997. [Google Scholar]
- 24.Austin PC, Walraven Cv. The Mortality Risk Score and the ADG Score: two points-based scoring systems for the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care. 2011;49:940–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barbera L, Hwee J, Klinger C, et al. Identification of the physician workforce providing palliative care in Ontario using administrative claims data. CMAJ Open. 2015;3:E292–E298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wodchis W, Bushmeneva K, Nikitovic M, et al. University of Toronto. Guidelines on Person Level Cost Using Administrative Databases in Ontario. Toronto, Ontario, Canada: University of Toronto; 2013. [Google Scholar]
- 27.Tanuseputro P, Budhwani S, Bai YQ, et al. Palliative care delivery across health sectors: a population-level observational study. Palliat Med. 2016;Published: 2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foebel AD, Hirdes JP, Lemick R, et al. Comparing the characteristics of people living with and without HIV in long-term care and home care in Ontario, Canada. AIDS Care. 2015;27:1343–1353. [DOI] [PubMed] [Google Scholar]
- 29.Foebel AD, Hirdes JP, Boodram C, et al. Comparing the care needs of people living with and without HIV in Canadian home and long-term care settings. Can Comm Dis Rep. 2016;42:52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson DM, Cohen J, Deliens L, et al. The preferred place of last days: results of a representative population-based public survey. J Palliat Med. 2013;16:502–508. [DOI] [PubMed] [Google Scholar]
- 31.Gomes B, Calanzani N, Gysels M, et al. Heterogeneity and changes in preferences for dying at home: a systematic review. BMC Palliat Care. 2013;12:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhodes RL, Nazir F, Lopez S, et al. Use and predictors of end-of-life care among HIV patients in a safety net health system. J Pain Symptom Manage. 2015;51:120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa V, Earle CC, Esplen MJ, et al. The determinants of home and nursing home death: a systematic review and meta-analysis. BMC Palliat Care. 2016;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simms V, Higginson IJ, Harding R. Integration of palliative care throughout HIV disease. Lancet Infect Dis. 2012;12:571–575. [DOI] [PubMed] [Google Scholar]
- 35.Merlins JS, Tucker RO, Saag MS, et al. The role of palliative care in the current HIV treatment era in developed countries. Top Antivir Med. 2013;21:20–26. [PMC free article] [PubMed] [Google Scholar]
- 36.Farber EW, Marconi VC. Palliative HIV care: opportunities for biomedical and behavioral change. Curr HIV/AIDS Rep. 2014;11:404–412. [DOI] [PubMed] [Google Scholar]
- 37.Wallach I, Brotman S. Ageing with HIV/AIDS: a scoping study among people aged 50 and over living in Quebec. Ageing Soc. 2012;1–31. [Google Scholar]
- 38.Buchanan RJ, Wang S, Huang C. Profiles of nursing home residents with HIV. J Health Care Poor Underserved. 2002;13:379–391. [DOI] [PubMed] [Google Scholar]
- 39.Lofgren S, Friedman R, Ghermay R, et al. Integrating early palliative care for patients with HIV: provider and patient perceptions of symptoms and need for services. Am J Hosp Palliat Med. 2015;32:829–834. [DOI] [PubMed] [Google Scholar]
- 40.Tanuseputro P, Chalifoux M, Bennett C, et al. Hospitalization and mortality rates in long-term care facilities: does for-profit status matter? J Am Med Dir Assoc. 2015;16:874–883. [DOI] [PubMed] [Google Scholar]
- 41.Harding R, Easterbrook P, Higginson IJ, et al. Access and equity in HIV/AIDS palliative care: a review of the evidence and responses. Palliat Med. 2005;19:251–258. [DOI] [PubMed] [Google Scholar]
- 42.Mosack KE, Wandrey RL. Discordance in HIV-positive patient and health care provider perspectives on death, dying, and end-of-life care. Am J Hosp Palliat Med. 2015;32:161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chai H, Guerriere DN, Zagorski B, et al. The magnitude, share and determinants of unpaid care costs for home-based palliative care service provision in Toronto, Canada. Health Soc Care Community. 2014;22:30–39. [DOI] [PubMed] [Google Scholar]
- 44.Choi SKY, Boyle E, Cairney J, et al. Adequacy of mental health services for HIV-positive patients with depression: Ontario HIV Treatment Network Cohort Study. PLoS One. 2016;11:e0156652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Advancing High Quality Palliative Care in Ontario: A Declaration of Partnership and Commitment to Action. 2011. Available online at: http://hpco.ca/HPCO_Advancing_High_Quality,_High_Value_Report.pdf. Accessed December 23, 2016. [Google Scholar]


