Abstract
In the last few years, neuroscientists have begun to identify associations between individual differences in decision-making and features of neuroanatomy and neurophysiology. Different tendencies in decision making, such as tolerance for risk, delay or effort, have been linked to various neurobiological measures, such as morphometry, structural connectivity, functional connectivity or the function of neurotransmitter systems. Though far from immutable, these neural features may nonetheless be suitable as relatively stable biomarkers for different decision traits. The establishment of such markers would achieve one of the stated goals of neuroeconomics, which is to improve the prediction of economic behavior across different contexts.
Predicting Decisions
Several neuroeconomists have argued that neural measures may aid in predicting behavior, and even skeptics of neuroeconomics agree that improved behavioral predictions—if they arrive—would constitute a contribution of neuroscience to economics [1–4]. Neuroeconomic studies have already shown that neural activity can improve predictions of simultaneous behavior and can predict later choices involving the same stimuli [5–8]. However, predictions of behavior that are farther from the context where the data were collected, in terms of stimuli or time, would be even more impressive and also more likely to be of practical use to economic questions [9].
One such practical purpose is to identify types of decision-makers [10,11]. Identifying and characterizing stable individual differences would aid in predicting individual-level behavior across many contexts. Properly accounting for such heterogeneity would also enable better macro-level predictions. For example, the outcomes of policy changes may differ depending on the composition of the population.
It is in the context of this potential promise of neuroeconomics that recent work identifying differences in brain structure and function at “rest” (i.e., without asking the subject to perform any task) is particularly interesting. Different forms of structural and functional imaging have found individual differences in morphometry, structural and functional connectivity, or resting neural activity [12–14]. Such neural differences, because of how they are measured and because of the features of the brain they reflect, are likely to be less tied to a specific context and fairly stable over time. Therefore, these neural measures may be well-suited to identifying relatively stable individual differences in decision making that predict behavior across many different behavioral contexts. In other words, though still early in development, these neuroscience tools could prove to be very useful for the goals of economists and other behavioral scientists.
Studies examining the relationship between these measures and cognitive ability have already been reviewed, and this literature serves as a nice example of both the promises and caveats of these techniques [15,16]. Here we review studies that have used these techniques to identify neural markers of individual differences in decision-making. We focus specifically on four different kinds of measures: measures of cortical thickness, gray and white matter density and volume from structural magnetic resonance imaging (MRI); measures of structural connectivity and white matter integrity from diffusion-tensor imaging (DTI); measures of resting functional connectivity from functional MRI (fMRI); and positron emission tomography (PET) measures of neurotransmitter transporters and receptors.
Morphometry
MRI can measure the structure of different brain regions and distinguish different tissue types such as grey matter, white matter and cerebrospinal fluid. Statistical techniques can be used to calculate the surface area or cortical thickness of a particular region of the cortical sheet, or the volume of grey or white matter at a particular location in standardized brain space. These measurements can then be related, across participants, to individual differences in behavior [12]. Although structural measures are thought to strongly rely on the specific properties of the scanner and scanning sequence used, recent research has demonstrated high test-retest reliability even between different scanning sites [17]. An oft-used statistical technique in this general family is called voxel-based morphometry (VBM), which tests for associations across individuals with grey and white matter volumes throughout the brain [18].
We have previously used VBM to examine the neuroanatomical correlates of risk attitudes (Figure 1) [19]. We first characterized behaviorally each participant’s risk tolerance, by using choices between gambles to estimate the curvature of their utility function for money in an expected-utility model. In an initial sample, greater risk tolerance was associated with increased grey matter volume in posterior parietal cortex, a region previously linked to decision making under risk in both human and non-human primate studies [20,21]. We then replicated this association in a second sample, demonstrating the parietal grey matter volume was a significant predictor of risk tolerance, over and above demographic variables (age and sex).
Figure 1.
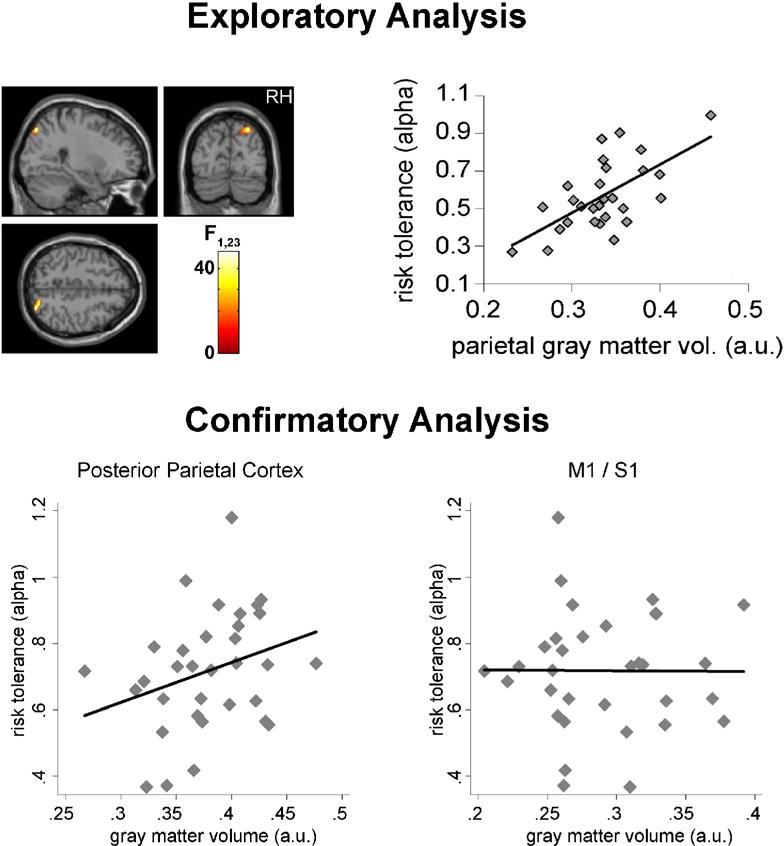
The cortical volume of a region in posterior parietal cortex is predictive of individual risk attitudes. Top: exploratory analysis revealed a significant positive correlation between the volume of the highlighted region (left) and risk tolerance (right) in a group of 28 individuals. Bottom: the result was confirmed in an independent group of participants, using a different scanner and a different behavioral task to estimate risk attitudes. Gray matter volume in the same region significantly predicted risk tolerance (p < 0.05; left), while the volume of a control region in the vicinity of the primary motor/somatosensory cortex was not predictive of risk attitudes (right). Adapted from [19].
Several studies have also examined the relationship between anatomical structure and delay discounting, the tendency to discount delayed rewards relative to immediate ones. The extent of discounting is typically characterized by the discount rate, which is estimated from choices between immediate and delayed rewards. The structure of prefrontal regions, the basal ganglia and the medial temporal lobe have all been implicated in discounting, though these associations are inconsistent across studies. Using VBM or other volumetric approaches, four studies examined single, relatively small, samples (n = 13–34) and focused on specific regions of interest. Bjork and colleagues (2009) found that greater grey matter volume in lateral prefrontal cortex was associated with reduced discount rates [22]. Cho and colleagues (2013) found that greater grey matter volume in medial prefrontal regions was associated with increased discount rates, while greater grey matter volume in putamen was associated with decreased discount rates [23]. Dombrovski and colleagues (2012) observed a similar association in the putamen in a sample of elderly suicide attempters, though they did not see the same association in a comparison sample of elderly depressed [24]. Using a task where the immediate reward was presented visually and the delayed reward verbally (therefore requiring visualization), Lebreton and colleagues (2013) found an association between greater hippocampal volume and reduced discounting; this association was specific to that condition and did not hold when the two rewards were presented in the same format [25]. Unfortunately, the two studies that have performed whole brain volumetric searches in larger samples (n > 100) have also yielded mixed results. In a combined sample of healthy and methamphetamine-dependent individuals, greater discounting was associated with increased grey matter volume in posterior cingulate and putamen and decreased grey matter volume in superior frontal gyrus [26]. A similar study in a completely healthy sample did not find any associations with grey matter volume, but did find associations with prefrontal and medial temporal white matter volumes [27]. The two studies using surface-based morphometric approaches are similarly mixed. Bernhardt and colleagues (2014) found that greater discounting was associated with decreased cortical thickness in an area of medial prefrontal cortex [28] defined from a previous fMRI study on delay discounting [29]. However, Drobetz and colleagues (2014) did not find any associations at corrected whole-brain thresholds [30], though they observed some associations in the lateral and medial prefrontal regions when applying a liberal threshold (p<0.05 uncorrected). Whether these inconsistencies in associations across studies are due to issues of statistical power, to differences of the applied morphometric techniques, or to true heterogeneity in the effects across different populations is unclear.
Structural Connectivity
Other aspects of neuroanatomical structure can be assessed with diffusion tensor imaging (DTI) [31,32]. DTI measures the diffusion of water molecules. In grey matter or CSF, this diffusion occurs uniformly in all directions, but in white matter, this diffusion is restricted by the directionality of the fiber pathways containing the axonal connections between brain regions. DTI measures the diffusion tensor, the extent of diffusion in each direction, in each point in space. Common summary properties that can be calculated include fractional anisotropy (FA), an index of the non-uniformity of diffusion, and mean diffusivity (MD), a measure of mean diffusion across all directions. Higher FA and lower MD are generally associated with greater white matter “integrity”. The diffusion tensor in each voxel can also be used to deterministically or probabilistically reconstruct the fiber pathways between brain regions. Similar to morphological measures, DTI measures show high test-retest reliability [33].
The integrity of connections between the frontal cortex and striatum has been linked to reduced delay discounting and to improved learning from rewards and punishments. Using probabilistic tractography, van den Bos and colleagues (2014) found that the extent of delay discounting was negatively correlated with the strength of tracts from the dorsolateral prefrontal cortex to striatum and positively correlated with the strength of tracts from the amygdala to striatum [34]. These findings held both in an original sample and a replication sample, and the strengths of these tracts were further associated with strength of functional connectivity during an intertemporal choice paradigm. The corticostriatal finding is consistent with a previous study that investigated the integrity of the entire frontal cortical-striatal tract and found an association between higher FA and lower MD in this tract and lower discounting [35], as well as with an earlier voxel-based study that found an association between lower discounting and higher FA and lower MD in white matter underlying right lateral prefrontal regions [36].
The integrity of another corticostriatal tract, from medial prefrontal cortex to ventral striatum, has been associated with age-related individual differences in learning (Figure 2) [37]. In this study, participants had to learn to select the stimulus with a greater probability of reward or with a lesser probability of punishment. Participants differed in their ability to learn these associations, and younger participants exhibited better learning. Greater FA within the tract from the dorsomedial thalamus to the medial prefrontal cortex and within the tract from the medial prefrontal cortex to the ventral striatum was associated with better learning, and the integrity of these tracts fully mediated the effects of age on learning.
Figure 2.
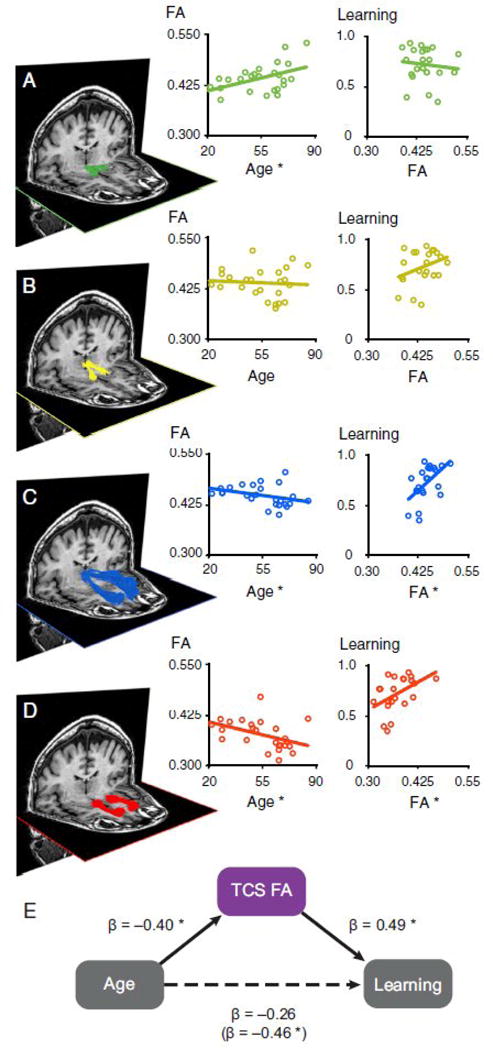
White matter tracts associated with age and learning. (A–D) White matter tracts in a representative subject between the VTA-NAcc (A), NAcc-DMThal (B), DMThal-MPFC (C), and MPFC-NAcc (D) and corresponding scatter plots across participants. The thalamocortical (C) and corticostriatal (D) tracts were associated with both age (N = 25) and learning (N = 22). (E) A combined measure of thalamocorticostriatal (TCS) white matter integrity mediated age differences in reward learning. Reprinted from [37].
Functional connectivity
In addition to anatomical markers, functional neural properties can also be predictive of individual personality traits and cognitive abilities. In the last two decades, blood oxygenation level dependent (BOLD) functional MRI has been increasingly used to assess intrinsic functional connectivity patterns [38]. Intrinsic functional connectivity is typically examined during “resting-state” fMRI scans, in which participants lie passively in the MRI scanner, are not exposed to external stimulation and are not asked to perform any task. The technique is based on the observation that spontaneous fluctuations in the BOLD signal result from intrinsic neuronal activity [39] and that these fluctuations exhibit spatial correlation patterns that largely reflect the underlying structural connectivity [40]. The test-retest reliability of resting-state functional connectivity is not fully known, but is expected to be lower than that of the structural measures. This is because in addition to scanner noise, functional measures are highly sensitive to physiological noise, including cardiac, respiration and head-motion artifacts. Still, recent examinations have shown moderate to high reliability of several resting states measures [41,42].
Recent studies have examined the possible associations between functional connectivity and individual decision traits. The common approach to these questions is to compute the coherence between signals obtained from predefined regions of interest, or between a particular “seed” region and the rest of the brain, during spontaneous activity [13]. The regions of interest are typically selected based on their involvement in decision-making processes, as inferred from task-based fMRI studies. The computed coherence measurements are then examined for potential associations with behavioral characteristics that are measured outside of the scanner in experimental tasks or based on self-report questionnaires.
Using this approach, several studies have identified associations between functional connectivity measures and discounting of future rewards (Figure 3). These studies documented a positive correlation between the degree of delay discounting and the strength of functional connectivity among components of the valuation network [43,44]. Such positive association was observed with the connectivity between the ventral striatum (VS) and the ventromedial prefrontal cortex (vmPFC) both in adults [43] and in children [45]. Positive association was also observed with the functional connectivity between the dorsal anterior cingulate cortex (dACC) and the anterior insula [43], between dACC and dopaminergic midbrain structures [46], and between fronto-insular cortex and vmPFC [47]. Conversely, there is evidence for negative correlation between delay discounting and the functional connectivity among lateral parietal and prefrontal regions, which have been implicated in the choice process [43]. While these findings cannot inform us about causal direction, they are consistent with the notion that weakened coupling between regions that have been implicated in self-control, such as the dACC and lateral prefrontal cortex (lPFC), and reward-related structures may increase preference for immediate rewards.
Figure 3.
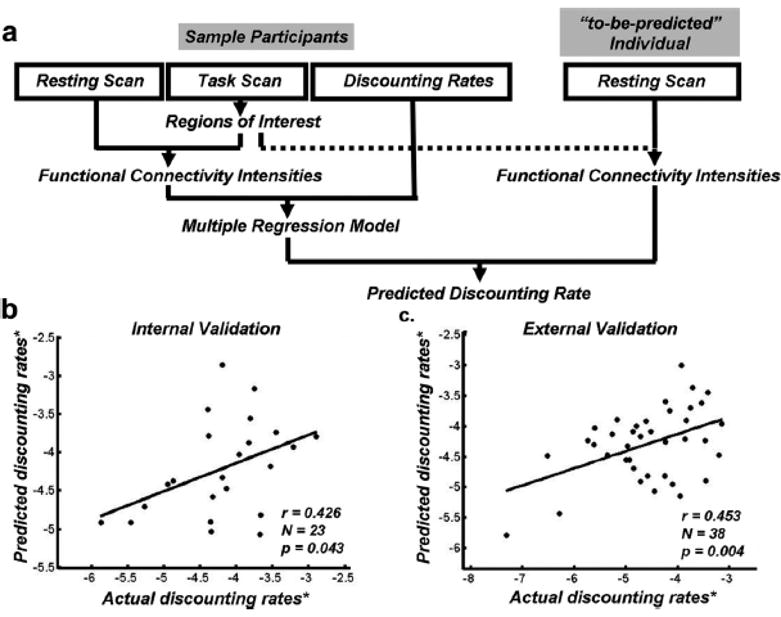
Resting state functional connectivity predicts discount rates. (a) Leave-one-out analysis was used, in which the resting state data of the left-out individual were calculated in the brain regions derived from networks defined from other participants to predict the discounting rate of the left-out individual. (b) Correlation between actual and predicted discount rates based on the leave-one-out analysis. (c) Confirmation in an independent new sample. * indicates log transformed. Reprinted from [43].
Positron emission tomography (PET)
Finally, positron emission tomography (PET) is also used to examine possible associations between brain function and behavioral traits. Using radioactive tracers PET imaging can track the distribution of various chemical compounds in different regions of the brain. This allows researchers to estimate the levels of specific neurotransmitters that are thought to be involved in particular behaviors. In the context of decision-making, dopamine, which has been implicated in reward and motivation, and specifically in encoding reward prediction errors that drive reinforcement learning [48] is of especially high interest. While dopamine levels cannot be directly measured, PET studies of dopamine rely on indirect measures, usually by labeling dopamine receptors, dopamine transporters or precursors of dopamine [49].
As can be expected, higher baseline striatal dopamine synthesis was associated with relatively better learning from unexpected rewards compared to unexpected punishments [50] as well as with better working memory [51]. Several studies have used the binding potential of [18F]fallypride, a D2/D3 selective ligand that labels striatal and extrastriatal receptors, as an indicator for receptor availability, a technique that has shown high test-retest reliability [52]. These studies have also utilized oral administration of d-amphetamine (AMPH), comparing receptor availability on AMPH and placebo as a measure for AMPH-induced dopamine release. In one of these studies, impulsivity measured by a widely-used self-report questionnaire (the Barratt Impulsiveness Scale, BIS-11; [53]), was negatively correlated with receptor availability in the substantia nigra/ventral tegmental area and positively correlated with the induced dopamine release in the striatum [54].
Treadway and colleagues [55] extended this approach to examine cost-benefit decision-making, where participants were asked to choose between exerting low physical effort for a small reward or high physical effort for a larger reward (Figure 4). They found that individual differences in dopamine function in left striatum and vmPFC were correlated with a willingness to expend more effort for larger rewards, especially when the reward probability was low, while variability in dopamine responses in bilateral insula negatively correlated with willingness to expend effort.
Figure 4.
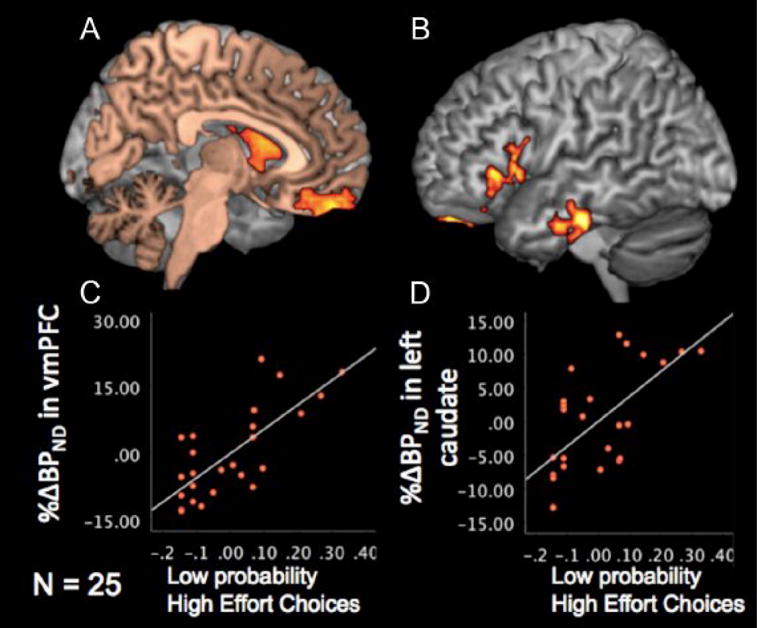
DA responses predict proportion of high effort choices. (A–B) Highlighted voxels showed significant positive correlation between proportion of high-effort choices during low-probability trials and DA responses in left caudate and vmPFC (A), as well as left vlPFC and temporal cortex. (C–D) Scatter plot of proportion of high-effort choices during low-probability trials and DA responses in vmPFC (C) and left caudate (D). Reprinted from [55].
Challenges and Future Directions
In interpreting all of these results, it is important to remember two caveats. First, all of the studies we have reviewed are cross-sectional. This means that we do not know how these neuroanatomical or neurophysiological differences developed. We do not know whether these differences preceded differences in decision making or followed from them (i.e., the association is consistent with both directions of causality), and we certainly do not know the extent to which these differences are innate, fixed or unchangeable. The existing data suggest that all of the neural measures we have discussed are malleable and can change with experience [56–62], and that their relationship with behavior can exhibit a complex developmental timecourse [63].
Second, many of the findings we have discussed above have yet to be independently replicated. There is growing recognition of the importance of replicability, in psychology and in the biological sciences in general [64–66]. For the goal of prediction, identifying neurobiological markers that are both replicable and generalizable across samples will be critical. This goal will be speeded as investigators increasingly collect and build large datasets. Functional connectivity datasets with sample sizes in the hundreds already exist [38] and the human connectome project aims to collect a similarly large sample of structural (T1) and structural connectivity (DTI) measures [67].
The findings reviewed here do provide “proof-of-principle” that neurobiological correlates of individual differences in decision-making can be identified, and we are optimistic that at least some of these differences will prove both reliable and generalizable. As this research area matures, we see at least two priorities. First, a wider variety of decision-making dimensions should be investigated. For example, differences in risk and ambiguity aversion, loss aversion, social preferences, strategic reasoning, and many other areas have yet to be fully explored. Second, few investigators have formally shown that brain metrics can predict decision behavior [19,43] and no one (to our knowledge) has yet shown that task-independent brain metrics can improve the prediction of decision-making over-and-above behavioral measures alone. This is especially important given the relative cost of neuroimaging compared to traditional behavioral measures (though in some cases the neuroimaging measures may already be available). A few recent studies have shown that task-related functional brain activation can improve prediction over the current behavioral “gold standard”, for example in the context of the persuasive messaging [68]. Only when this last criterion has been reached will neuroeconomists have achieved the goal of improving economic prediction.
Highlights.
Individual differences in decision-making are linked to aspects of neuroanatomy and neurophysiology.
Neurobiological features could be suitable as biomarkers for decision traits.
Neural markers could aid in prediction of economic behavior.
Acknowledgments
JWK was supported by NIH grant R01DA029149. IL was supported by NIH grants R01AG033406 and R21MH102634.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Camerer CF. Neuroeconomics: Using Neuroscience to Make Economic Predictions*. The Economic Journal. 2007;117:C26–C42. [Google Scholar]
- 2.Camerer CF. The potential of neuroeconomics. Economics and Philosophy. 2008;24:369–379. [Google Scholar]
- 3.Kable JW. Neuroeconomics: How Neuroscience Can Inform the Social Sciences. In: Sun R, editor. Grounding Social Sciences in Cognitive Sciences. The MIT Press; 2012. pp. 315–350. [Google Scholar]
- 4.Bernheim BD. On the Potential of Neuroeconomics: A Critical (but Hopeful) Appraisal. American Economic Journal: Microeconomics. 2009;1:1–41. [Google Scholar]
- 5.Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levy I, Lazzaro SC, Rutledge RB, Glimcher PW. Choice from non-choice: predicting consumer preferences from blood oxygenation level-dependent signals obtained during passive viewing. J Neurosci. 2011;31:118–125. doi: 10.1523/JNEUROSCI.3214-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tusche A, Bode S, Haynes J-D. Neural responses to unattended products predict later consumer choices. J Neurosci. 2010;30:8024–8031. doi: 10.1523/JNEUROSCI.0064-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith A, Bernheim BD, Camerer CF, Rangel A. Neural Activity Reveals Preferences without Choices. American Economic Journal: Microeconomics. 2014;6:1–36. doi: 10.1257/mic.6.2.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fumagalli R. Neural Findings and Economic Models: Why Brains Have Limited Relevance for Economics. Philosophy of the Social Sciences. 2014;44:606–629. [Google Scholar]
- 10.Houser D, Schunk D, Xiao E. Combining brain and behavioral data to improve econometric policy analysis. Analyse & Kritik. 2007;29:86–96. [Google Scholar]
- 11.Spiegler R. Comments on the potential significance of neuroeconomics for economic theory. Economics and Philosophy. 2008;24:515–521. [Google Scholar]
- 12.Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- 13.Vaidya CJ, Gordon EM. Phenotypic variability in resting-state functional connectivity: current status. Brain Connect. 2013;3:99–120. doi: 10.1089/brain.2012.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kable JW. The cognitive neuroscience toolkit for the neuroeconomist: A functional overview. Journal of Neuroscience, Psychology, and Economics. 2011;4:63–84. doi: 10.1037/a0023555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray JR, Thompson PM. Neurobiology of intelligence: science and ethics. Nat Rev Neurosci. 2004;5:471–482. doi: 10.1038/nrn1405. [DOI] [PubMed] [Google Scholar]
- 16.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- 17.Schnack HG, van Haren NEM, Brouwer RM, van Baal GCM, Picchioni M, Weisbrod M, Sauer H, Cannon TD, Huttunen M, Lepage C, et al. Mapping reliability in multicenter MRI: voxel-based morphometry and cortical thickness. Hum Brain Mapp. 2010;31:1967–1982. doi: 10.1002/hbm.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. NeuroImage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 19••.Gilaie-Dotan S, Tymula A, Cooper N, Kable JW, Glimcher PW, Levy I. Neuroanatomy Predicts Individual Risk Attitudes. Journal of Neuroscience. 2014;34:12394–12401. doi: 10.1523/JNEUROSCI.1600-14.2014. Using voxel-based morphometry, the authors demonstrate an association between risk tolerance and grey matter volume in the parietal cortex. In replication sample, they show that parietal grey matter volume significantly improves the prediction of risk attitudes above demographic variables. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400:233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 21.Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–775. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 22.Bjork JM, Momenan R, Hommer DW. Delay discounting correlates with proportional lateral frontal cortex volumes. Biol Psychiatry. 2009;65:710–713. doi: 10.1016/j.biopsych.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Cho SS, Pellecchia G, Aminian K, Ray N, Segura B, Obeso I, Strafella AP. Morphometric correlation of impulsivity in medial prefrontal cortex. Brain Topogr. 2013;26:479–487. doi: 10.1007/s10548-012-0270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dombrovski AY, Siegle GJ, Szanto K, Clark L, Reynolds CF, Aizenstein H. The temptation of suicide: striatal gray matter, discounting of delayed rewards, and suicide attempts in late-life depression. Psychol Med. 2012;42:1203–1215. doi: 10.1017/S0033291711002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lebreton ML, Bertoux M, Boutet C, Lehericy SP, Dubois B, Fossati P, Pessiglione M. A Critical Role for the Hippocampus in the Valuation of Imagined Outcomes. PLoS Biol. 2013;11:e1001684. doi: 10.1371/journal.pbio.1001684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schwartz DL, Mitchell AD, Lahna DL, Luber HS, Huckans MS, Mitchell SH, Hoffman WF. Global and local morphometric differences in recently abstinent methamphetamine-dependent individuals. NeuroImage. 2010;50:1392–1401. doi: 10.1016/j.neuroimage.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu R. Regional white matter volumes correlate with delay discounting. PLoS ONE. 2012;7:e32595. doi: 10.1371/journal.pone.0032595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernhardt BC, Smallwood J, Tusche A, Ruby FJM, Engen HG, Steinbeis N, Singer T. Medial prefrontal and anterior cingulate cortical thickness predicts shared individual differences in self-generated thought and temporal discounting. NeuroImage. 2014;90:290–297. doi: 10.1016/j.neuroimage.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10:1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Drobetz R, Hänggi J, Maercker A, Kaufmann K, Jäncke L, Forstmeier S. Structural brain correlates of delay of gratification in the elderly. Behav Neurosci. 2014;128:134–145. doi: 10.1037/a0036208. [DOI] [PubMed] [Google Scholar]
- 31.Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Johansen-Berg H, Rushworth MFS. Using diffusion imaging to study human connectional anatomy. Annu Rev Neurosci. 2009;32:75–94. doi: 10.1146/annurev.neuro.051508.135735. [DOI] [PubMed] [Google Scholar]
- 33.Buchanan CR, Pernet CR, Gorgolewski KJ, Storkey AJ, Bastin ME. Test-retest reliability of structural brain networks from diffusion MRI. NeuroImage. 2014;86:231–243. doi: 10.1016/j.neuroimage.2013.09.054. [DOI] [PubMed] [Google Scholar]
- 34••.van den Bos W, Rodriguez CA, Schweitzer JB, McClure SM. Connectivity strength of dissociable striatal tracts predict individual differences in temporal discounting. J Neurosci. 2014;34:10298–10310. doi: 10.1523/JNEUROSCI.4105-13.2014. Using diffusion-tensor imaging, this study finds that the strength of connections between dorsolateral prefrontal cortex and striatum predicts reduced delay discounting, while the strength of connections between the amygdala and striatum predicts increased delay discounting. These structural associations are replicated in a second sample and are correlated with functional connectivity to the striatum while individuals make intertemporal choices. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peper JS, Mandl RCW, Braams BR, de Water E, Heijboer AC, Koolschijn PCMP, Crone EA. Delay discounting and frontostriatal fiber tracts: a combined DTI and MTR study on impulsive choices in healthy young adults. Cereb Cortex. 2013;23:1695–1702. doi: 10.1093/cercor/bhs163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J Cogn Neurosci. 2009;21:1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37••.Samanez-Larkin GR, Levens SM, Perry LM, Dougherty RF, Knutson B. Frontostriatal white matter integrity mediates adult age differences in probabilistic reward learning. J Neurosci. 2012;32:5333–5337. doi: 10.1523/JNEUROSCI.5756-11.2012. This study shows that the integrity of thalamocortical and corticostriatal pathways is associated with the ability to learn from probabilistic rewards and punishments, and that these differences mediate the decremental effect of aging on reward learning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 40.Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran JP, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc Natl Acad Sci USA. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zuo X-N, Xing X-X. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neuroscience and Biobehavioral Reviews. 2014;45:100–118. doi: 10.1016/j.neubiorev.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 42.Shehzad Z, Kelly AMC, Reiss PT, Gee DG, Gotimer K, Uddin LQ, Lee SH, Margulies DS, Roy AK, Biswal BB, et al. The resting brain: unconstrained yet reliable. Cereb Cortex. 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••43.Li N, Ma N, Liu Y, He X-S, Sun D-L, Fu X-M, Zhang X, Han S, Zhang D-R. Resting-state functional connectivity predicts impulsivity in economic decision-making. J Neurosci. 2013;33:4886–4895. doi: 10.1523/JNEUROSCI.1342-12.2013. These authors show that resting functional connectivity measures can predict delay discounting behavior. Specifically, stronger connectivity between regions involved in reward valuation predicts increased discounting, while stronger connectivity between regions involved in choice and action predicts reduced discounting. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calluso C, Tosoni A, Pezzulo G, Spadone S, Committeri G. Interindividual variability in functional connectivity as long-term correlate of temporal discounting. PLoS ONE. 2015;10:e0119710. doi: 10.1371/journal.pone.0119710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, Stevens CA, Musser ED, Carpenter SD, Grayson DS, et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23:33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmaal L, Goudriaan AE, van der Meer J, van den Brink W, Veltman DJ. The association between cingulate cortex glutamate concentration and delay discounting is mediated by resting state functional connectivity. Brain Behav. 2012;2:553–562. doi: 10.1002/brb3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Han SD, Boyle PA, Yu L, Fleischman DA, Arfanakis K, Bennett DA. Ventromedial PFC, parahippocampal, and cerebellar connectivity are associated with temporal discounting in old age. Exp Gerontol. 2013;48:1489–1498. doi: 10.1016/j.exger.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 49.Volkow ND, Fowler JS, Gatley SJ, Logan J, Wang GJ, Ding YS, Dewey S. PET evaluation of the dopamine system of the human brain. J Nucl Med. 1996;37:1242–1256. [PubMed] [Google Scholar]
- 50.Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. J Neurosci. 2009;29:1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cools R, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci. 2008;28:1208–1212. doi: 10.1523/JNEUROSCI.4475-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee J, Christian BT, Dunigan KA, Shi B, Narayanan TK, Satter M, Mantil J. Brain imaging of 18F-fallypride in normal volunteers: blood analysis, distribution, test-retest studies, and preliminary assessment of sensitivity to aging effects on dopamine D-2/D-3 receptors. Synapse. 2002;46:170–188. doi: 10.1002/syn.10128. [DOI] [PubMed] [Google Scholar]
- 53.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 54.Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, et al. Dopaminergic network differences in human impulsivity. Science. 2010;329:532–532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. This study demonstrates the use of PET to identify individual differences in neural function. The authors find an association between the dopamine response in ventromedial prefrontal cortex and ventral striatum and the willingness to expend effort in contexts where there is a lower probability of reward. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- 57.Ilg R, Wohlschläger AM, Gaser C, Liebau Y, Dauner R, Wöller A, Zimmer C, Zihl J, Mühlau M. Gray matter increase induced by practice correlates with task-specific activation: a combined functional and morphometric magnetic resonance imaging study. J Neurosci. 2008;28:4210–4215. doi: 10.1523/JNEUROSCI.5722-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuhn S, Gleich T, Lorenz RC, Lindenberger U, Gallinat J. Playing Super Mario induces structural brain plasticity: gray matter changes resulting from training with a commercial video game. Mol Psychiatry. 2014;19:265–271. doi: 10.1038/mp.2013.120. [DOI] [PubMed] [Google Scholar]
- 59.Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white-matter architecture. Nature Publishing Group. 2009;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 61.Taubert M, Lohmann G, Margulies DS, Villringer A, Ragert P. Long-term effects of motor training on resting-state networks and underlying brain structure. NeuroImage. 2011;57:1492–1498. doi: 10.1016/j.neuroimage.2011.05.078. [DOI] [PubMed] [Google Scholar]
- 62.McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323:800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- 63.Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 64.Ioannidis JPA. Why Most Published Research Findings Are False. Plos Med. 2005;2:e124. doi: 10.1371/journal.pmed.0020124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14:365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- 66.Pashler H, Wagenmakers E-J. Editors’ Introduction to the Special Section on Replicability in Psychological Science A Crisis of Confidence? Perspectives on Psychological Science. 2012;7:528–530. doi: 10.1177/1745691612465253. [DOI] [PubMed] [Google Scholar]
- 67.Van Essen DC, Ugurbil K, Auerbach E, Barch D, Behrens TEJ, Bucholz R, Chang A, Chen L, Corbetta M, Curtiss SW, et al. The Human Connectome Project: A data acquisition perspective. NeuroImage. 2012;62:2222–2231. doi: 10.1016/j.neuroimage.2012.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berkman ET, Falk EB. Beyond Brain Mapping: Using Neural Measures to Predict Real-World Outcomes. Current Directions in Psychological Science. 2013;22:45–50. doi: 10.1177/0963721412469394. [DOI] [PMC free article] [PubMed] [Google Scholar]


