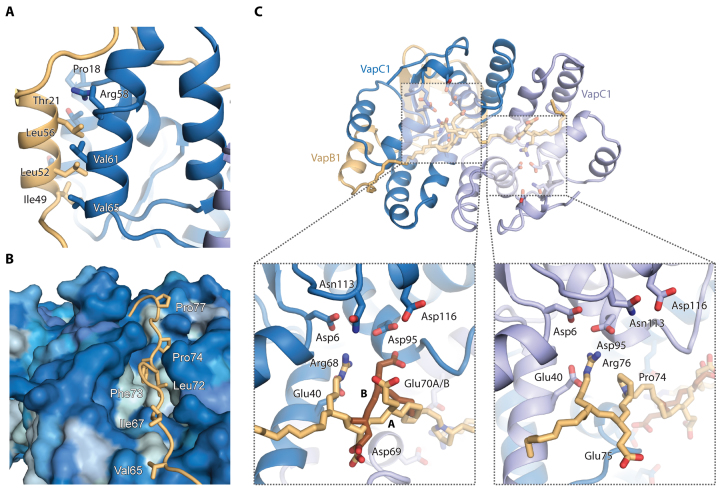
Figure 2.
Details of toxin–antitoxin interactions. (A) The lateral interaction site is dominated by hydrophobic interactions between VapB1 (light brown) and VapC1 (blue) in a leucine zipper-like motif. (B) At the groove site, the extended C-terminus of VapB1 (light brown) lies in a hydrophobic groove spanning the surface of the VapC1 dimer (blue). The VapC1 surface is coloured by hydrophobicity (light blue = hydrophobic, dark blue = hydrophilic) and relevant, interacting residues in VapB1 are labeled. (C) Overview of the 1:2 antitoxin:toxin interaction of the extended C-terminus of VapB1 (top) and close-up views of interactions at the two active sites of the VapC1 homodimer (bottom). Conserved acidic residues in the VapC1 active sites are shown in blue sticks and interacting residues in VapB1 with light brown sticks. VapB1 displays two discrete main chain conformations (A and B) at one site, which is illustrated with light/dark brown backbone colours.