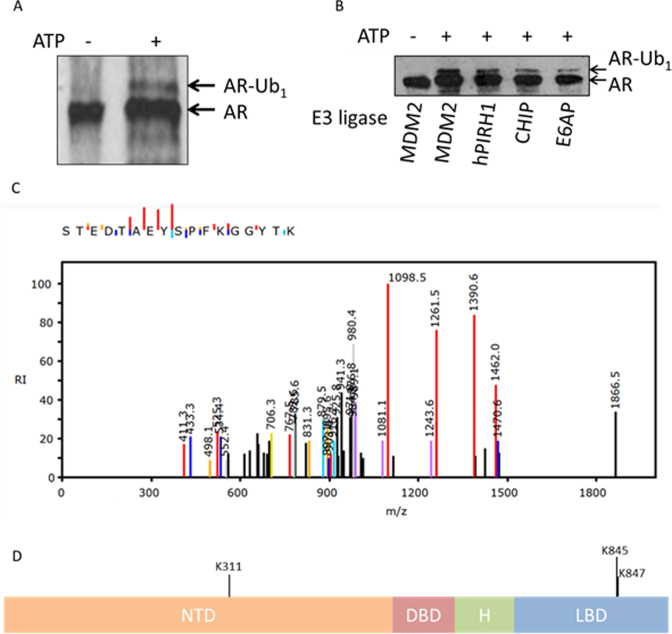
Figure 1.
In vitro identification of K311 as a site of AR ubiquitination. (A) In vitro monoubiquitination of AR by MDM2 upon addition of ATP. Monoubiquitinated AR (AR-Ub1) was excised from the colloidal-coomassie stained gel for MSMS analysis. (B) Monoubiquitination of AR-NTD protein (AR-Ub1) can be detected by Western blotting following in vitro ubiquitination reactions in the presence of ATP, UBE1, UBCH5α and UbK0 using four different E3 ligases; MDM2, hPIRH2, CHIP and E6AP. (C) MSMS spectrum of the tryptic peptide, <302>STEDTAEYSPFK*GGYTK<318>, observed following in gel tryptic digest. Analysis of the spectrum locates unequivocally a diglycine modification to K311. (D) AR domain structure highlighting the novel site of AR ubiquitination, K311, within the N-terminal domain (NTD). Previously identified RNF6 target lysines within the ligand binding domain (LBD) are also indicated, K845 and K847. DBD—DNA binding domain, H—hinge.