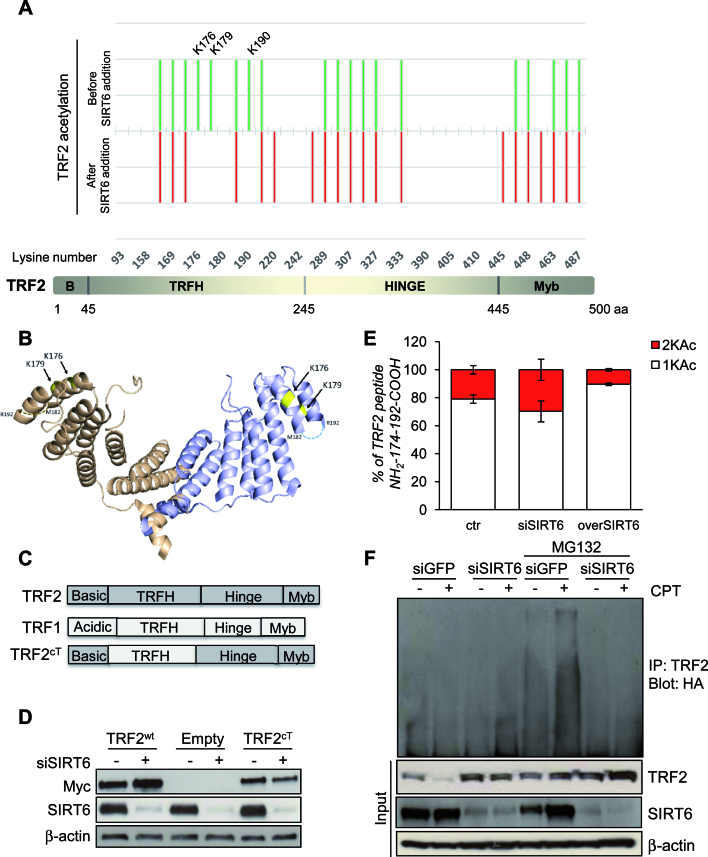
Figure 5.
TRF2 is deacetylated by SIRT6. (A) Lower panel: Representation of TRF2 organization in four domains. Upper panel: Footprinting graph showing profile of in vitro acetylation of TRF2 lysines by sulfosuccinimidyl acetate (in green) and TRF2 lysines still acetylated upon SIRT6 addition (in red). The mass spectrometry analysis was done using a nanoLC-MSMS giving both the peptide mass and sequence allowing the determination of the specific acetylated aminoacids. Mass spectrometry analysis gives acetylation profiles of the protein before and after incubation with SIRT6 showing TRF2 lysines deacetylated by SIRT6. (B) Positions of lysines deacetylated upon SIRT6 addition (in yellow), on the 3D structure of the TRFH domain of TRF2 (PDB:3BUA). The third lysine identified, K190 is located on a region of structural disorder, represented as broken lines. (C) Schematic representation of the TRF2cT variant obtained through the domain swapping approach between TRF1 and TRF2. (D) HCT116 cells infected with Myc-TRF2wt, Myc-TRF2cT or Empty retroviral vectors were analyzed by western blot for the expression of Myc-tagged TRF2 variants or SIRT6, after 72 h of siGFP or siSIRT6 transfection. (E) TRF2-overexpressing HCT116 cells were subjected to IP with anti-TRF2 antibody after silencing (siSIRT6) or overexpression (overSIRT6) of SIRT6. Immunoprecipitated TRF2 proteins were loaded on SDS-PAGE and the regions around 60 kDa were cut and, separately, digested as described in material and methods section, to be then analyzed by LC–MS/MS. The relative abundance of the ions of peptide 174–192 mono- (+1KAc) and diacetylated (+2KAc) species was calculated integrating the peak area in each LC–MSMS run and reported in the histograms. (F) After transient transfection with ubiquitin-HA vector, TRF2-overexpressing HCT116 cells were either transfected with siSIRT6 siRNA or control siGFP. Protein lysates prepared from cells after 6 h by the end of treatment with 2 μM CPT, as well as those from untreated cells as negative controls, in presence or not of MG132, were used in immunoprecipitation with anti-TRF2 antibody, followed by western blot with anti-HA. The effectiveness of SIRT6 siRNA and the expression of TRF2 was confirmed by immunoblot using input samples. The levels of β-actin were used as a loading control.