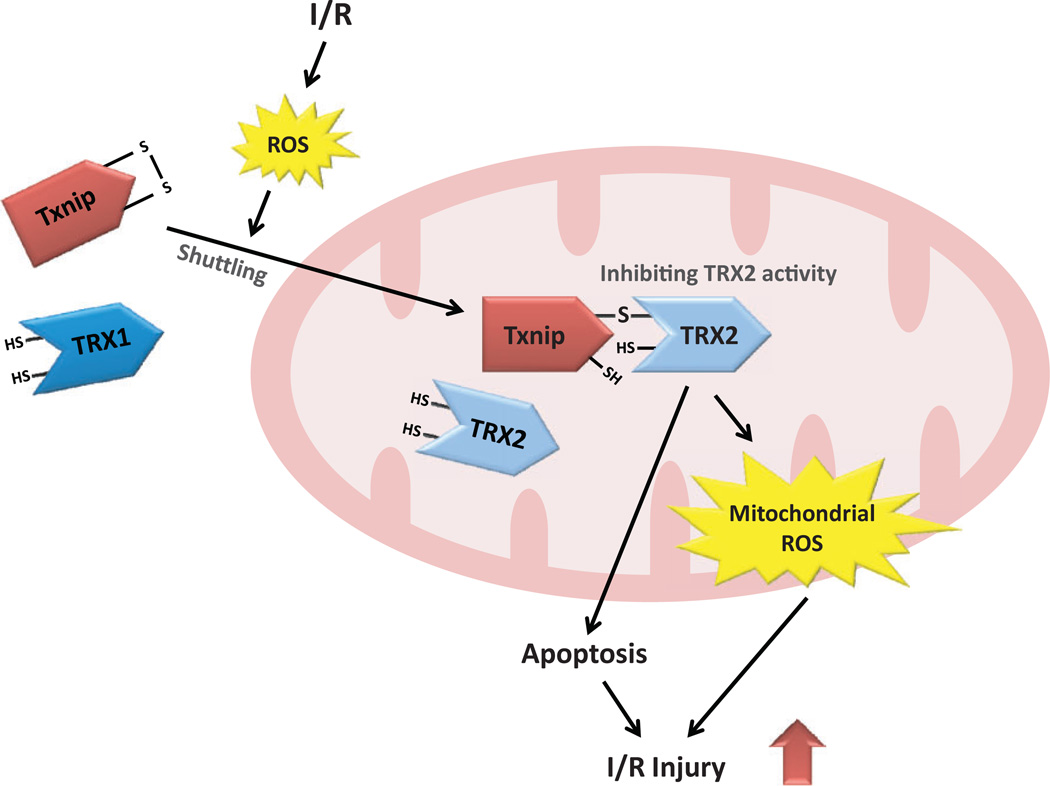
Figure 1.
Possible mechanism by which thioredoxin-interacting protein (Txnip) mediates ischemia/reperfusion (I/R) injury by interacting with mitochondrial thioredoxin-2 (TRX2). Ischemia/reperfusion induces cellular production of reactive oxygen species (ROS). In response to oxidative stress, Txnip is shuttled from the nucleus to mitochondria, where TRX2 regulates the generation of mitochondrial ROS and the mitochondrial apoptosis signaling pathway. Thioredoxin-2 also repairs reversible oxidative protein damage by reducing disulfide bonds that are formed by the oxidation of cysteines. Thioredoxin-interacting protein binds to TRX2 through a disulfide bond formation, inhibiting the reducing functions of TRX2. The interaction between Txnip and TRX2 promotes the generation of mitochondrial ROS and triggers the mitochondrial apoptosis signaling pathway, which leads to I/R injury.