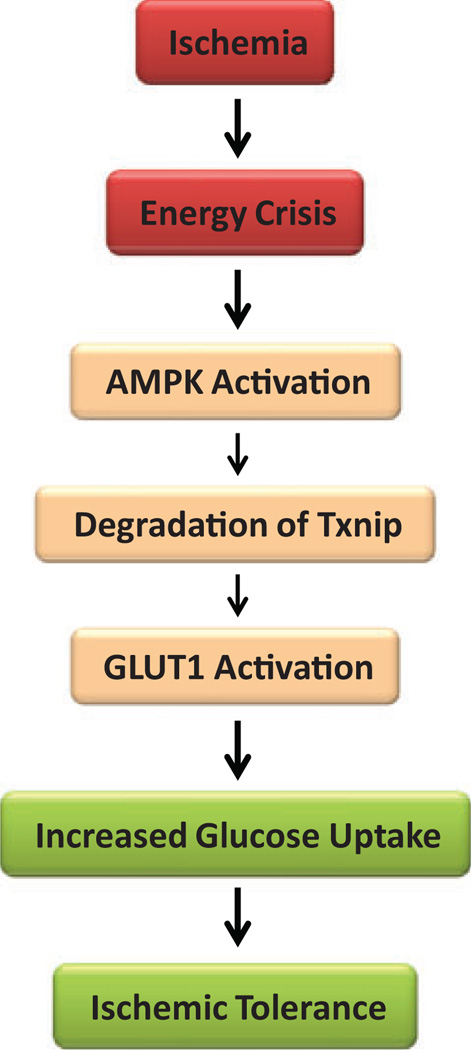
Figure 3.
A hypothetical mechanism of cardioprotection by suppressing thioredoxin-interacting protein (Txnip) during ischemia. Ischemia causes energy stress. The adenosine monophosphate-dependent protein kinase (AMPK) is a sensor of energy status that is activated in response to cellular energy crisis. The AMPK promotes the phosphor-ylation and rapid degradation of Txnip protein, which leads to the suppression of Txnip. Glucose transporter 1 (GLUT1) facilitates the transport of glucose into the cell and is responsible for basal glucose uptake required to sustain energy homeostasis. Thioredoxin-interacting protein binds to GLUT1 and inhibits cellular glucose uptake. Conversely, Txnip suppression results in increased GLUT1 function, which promotes glucose uptake during ischemia. Enhanced glucose uptake is considered to support anaerobic metabolism and confer ischemic tolerance.