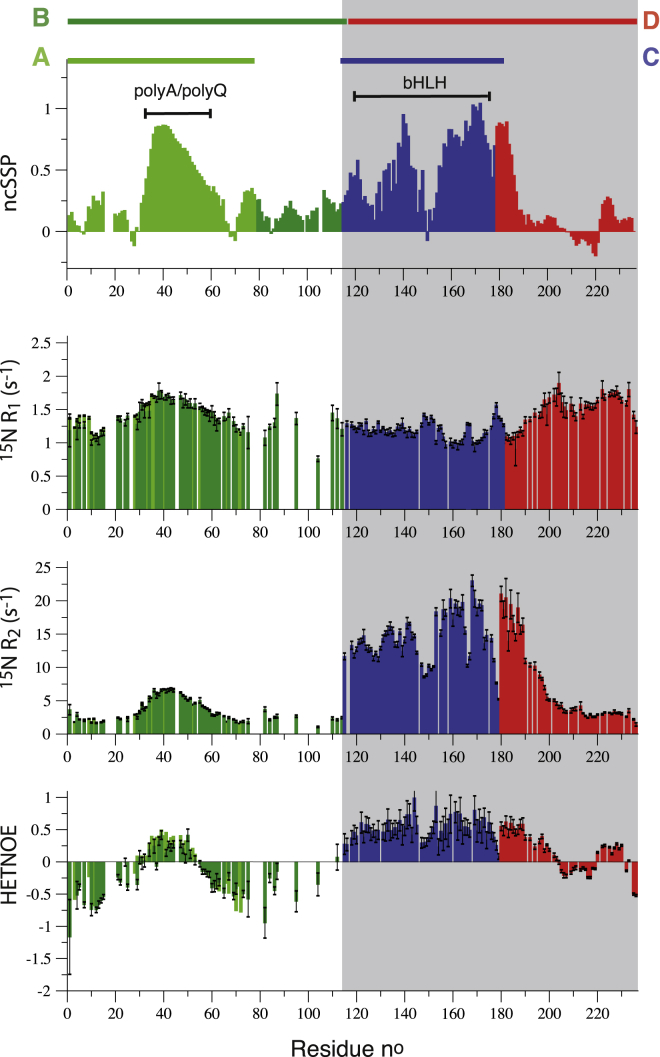
Figure 3.
NMR data collected for the four fragments of Ascl1, reporting on conformational features (structure and dynamics) along the polypeptide chain. From top to bottom are shown neighbor-corrected secondary structural propensity (ncSSP) values, calculated from the measured 13C chemical shifts, 15N R1 and R2 relaxation rate constants, and HETNOE intensity ratios. 15N relaxation data were recorded at 25°C, and the magnetic field strength was 600 MHz 1H frequency (700 MHz for fragment D). Data measured for the highly flexible terminal residues at the nonnative fragment ends are not shown. All graphs are color-coded to make clear that the underlying data originate from different NMR samples. White (grey) backgrounds indicate that the data have been measured on sampels without (with) detergent. To see this figure in color, go online.