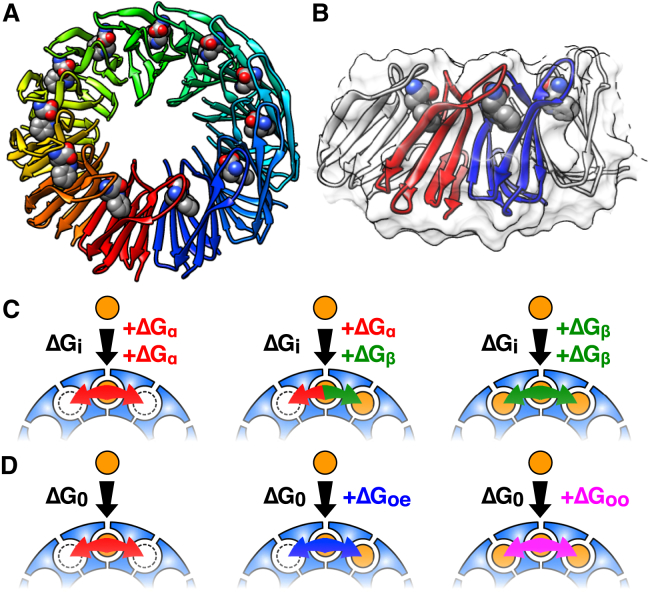
Figure 1.
TRAP is a biosensor with 11 equivalent binding sites for tryptophan. (A) Crystal structure of Trp-bound TRAP11 (26); bound Trp are shown as spheres. (B) Closeup of two neighboring protomers in the crystal structure (red and blue) showing proximity of bound tryptophan molecules. (C) NN-a model of cooperativity between sites, in which binding free energy is the sum of an intrinsic free energy ΔGi plus coupling to either empty (ΔGα) or occupied (ΔGβ) neighboring sites. (D) NN-na model, in which binding to a site flanked by two empty sites has a reference ΔG0, modified by coupling free energies when the site is flanked by one or two occupied neighbors (ΔGoe, ΔGoo), respectively. To see this figure in color, go online.