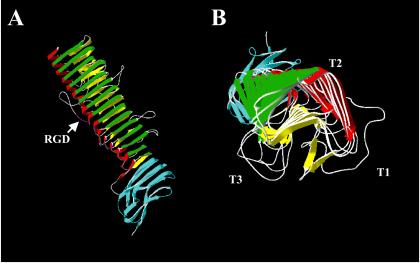
FIG. 11.
Crystal structure of the B. pertussis protein pertactin. The structure of pertactin was solved to a resolution of 2.5 Å (123). (A) Side view of pertactin, with the N terminus located to the top. (B) End view of pertactin looking from the N terminus toward the C terminus. This protein consists of 16 parallel β-strands arranged in a left-handed helix from which several loops extend. T1, T2, and T3 represent the turns between the three strands of the β-helix. The RGD motif is shown in purple in T1. The region in blue represents a conserved region that is present in many autotransporters and has been shown to mediate folding of the passenger domain (351). This is the largest β-helix known. Structurally, this molecule is related to the tail spike proteins of P22 phage and to the pectin lyases and methylesterases (227). Adapted from reference 123 and the Protein Data Bank (30).