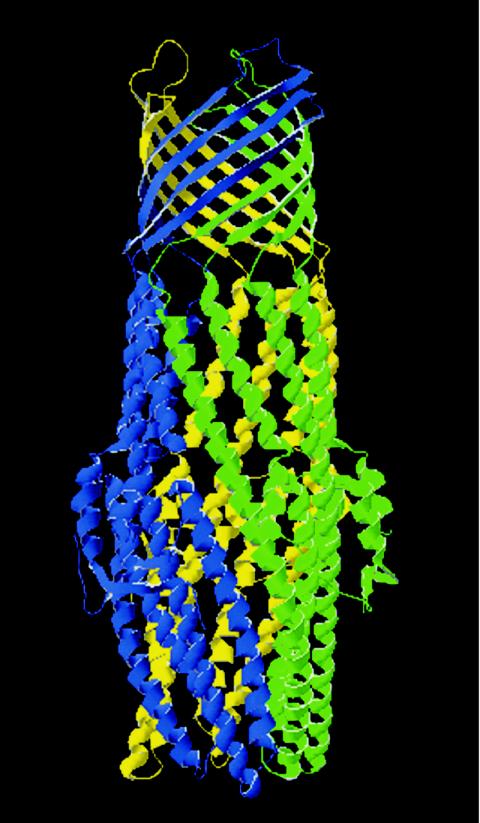
FIG. 2.
Structure of TolC. The structure of TolC was solved to 2.1-Å resolution. The functional TolC unit consists of three TolC monomers oligomerized into a regular structure embedded in the outer membrane and extending into the periplasm such that it forms a continuous solvent-accessible conduit. The portion of the trimeric molecule that is embedded in the outer membrane adopts a 12-strand β-barrel conformation consisting of four β-strands per monomer. The molecule is 140 Å long, the β-barrel is 40 Å long (spanning the outer membrane), and the remaining portion of the molecule adopts a 100 Å α-helical conformation spanning the periplasmic space. This α-helical domain consists of 12 α-helices, such that the α-helices extend from or connect to the periplasmic side of each β-strand. It is the α-helical domain that is predicted to interact with the MFP. Each monomer is highlighted in a separate color. Adapted from reference 263 and the Protein Data Bank (30).