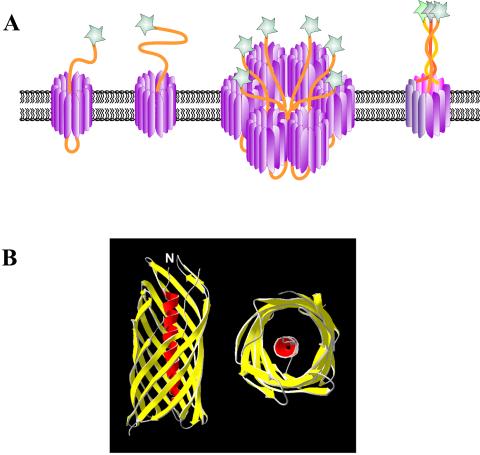
FIG. 5.
Alternative mechanisms of autotransporter protein secretion. (A) Several different hypotheses have been proffered for the mechanism of autotransporter protein biogenesis. From left to right are depicted the traditional view of secretion originally proposed by Pohlner et al. (401), in which the passenger domain passes through the pore of the β-barrel to the outside; a recently proposed structure where the passenger domain extends directly from the β-barrel into the extracellular milieu; the secretion pathway proposed by Veiga et al. (512), in which a central channel is formed through which the passenger domains are secreted to the outside, and the pathway proposed by Hoiczyk et al. (210) for the type Vc autotransporter family, in which several molecules contribute β-strands to make a large β-barrel pore through which the proteins are secreted to the external side of the outer membrane. (B) Crystal structure of the AspA/NalP translocating unit to 2.6 Å. A side view and a stereo-top view are depicted. The protein forms a 12-strand β-barrel structure characterized by short periplasmic turns and longer external loops. The barrel interior is highly hydrophilic due to the presence of charged amino acids. Within the barrel is embedded an α-helical region, which is attached to the first transmembrane β-strand such that the extreme N terminus of the protein, and to which it is presumed a native passenger domain would be attached, is located on the extracellular surface. Adapted from reference 366 and the Protein Data Bank (30).