Abstract
Acute kidney injury (AKI) is an independent risk factor for ensuing chronic kidney disease (CKD). Animal studies have demonstrated that renin-angiotensin system (RAS) inhibitor can reduce ensuing CKD after functional recovery from AKI. Here we study the association between ensuing CKD and use of RAS inhibitor including angiotensin converting enzyme inhibitor or angiotensin II type 1a receptor blocker starting after renal functional recovery in our prospectively collected observational AKI cohort. Adult patients who had cardiac surgery–associated AKI (CSA-AKI) are studied. Patients with CKD, unrecovered AKI, and use of RAS inhibitor before surgery are excluded. Among 587 eligible patients, 94 patients are users of RAS inhibitor which is started and continued after complete renal recovery during median follow-up period of 2.99 years. The users of RAS inhibitor show significantly lower rate of ensuing CKD (users vs. non-users, 26.6% vs. 42.2%) and longer median CKD-free survival time (users vs. non-users, 1079 days vs. 520 days). Multivariate Cox regression analyses further demonstrate that use of RAS inhibitor is independently associated with lower risk of ensuing CKD (hazard ratio = 0.46, P < 0.001). We conclude that use of RAS inhibitor in CSA-AKI patients after renal functional recovery is associated with lower risk of ensuing CKD development.
Acute kidney injury (AKI) is common and associated with higher morbidity and mortality globally1,2,3,4. AKI has also been recognized as a major risk factor for the development of chronic kidney disease (CKD)5,6. Mounting evidence has shown that AKI and CKD appear to be an interconnected syndrome7. The severity, duration and frequency of AKI has been linked to the development and progression of ensuing CKD6,7,8,9,10. Continuous monitoring of renal function has been emphasized, even if patients have shown functional recovery after AKI5,6. Many studies have focused on the prevention and management of AKI to reduce the ensuing CKD development11,12,13. Nevertheless, more efforts are needed to develop strategies for blocking AKI-CKD transition after functional recovery.
Although the mechanisms underlying AKI-CKD transition are incompletely understood in humans, animal studies have shown a number of pathogenetic mechanisms such as maladaptive repair14, profibrogenic cytokine production by G2/M cell-cycle arrested epithelia15, pericyte-myofibroblast transition16,17, and microvascular rarefaction18,19. These mechanisms open up opportunities to innovate therapeutic strategies for prevention of AKI-CKD transition. For AKI patients with incomplete recovery of renal function, we can treat them as CKD patients. However, we have neither consensus nor reliable therapeutic interventions for patients with renal functional recovery from AKI.
Recently, animal studies have demonstrated that activation of intrarenal renin–angiotensin system (RAS) after AKI underlies the possible mechanism for development and progression of ensuing CKD20,21,22. To get insight into the clinical application of RAS inhibitor and its impact on development of ensuing CKD in AKI survivors with complete renal recovery, we studied the outcomes and relevant risk factors of cardiac surgery–associated AKI (CSA-AKI) patients who did not have CKD history before surgery in our prospectively collected observational cohort.
Results
Baseline Characteristics and Follow-up for CKD Development
Of the 1117 patients who underwent cardiac surgery during the period of January 1, 2000 and December 31, 2011, 530 patients were excluded from analysis due to no AKI (103 patients), diagnosis of CKD or estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 before surgery (75 patients), unrecovered AKI (69 patients), follow-up less than 3 months after recovery from AKI (99 patients) and regular medication with RAS inhibitor including angiotensin converting enzyme (ACE) inhibitor or angiotensin II type 1a receptor blocker (ARB) before AKI (184 patients). Of the 587 eligible patients, 94 patients were users of RAS inhibitor which was started after complete renal recovery defined as the decrease of serum creatinine (SCr) level to within 0.3 mg/dl above the baseline and continued during the median follow-up period of 2.99 years (Fig. 1). The other 493 patients were non-users of RAS inhibitor.
Figure 1. Flow diagram of patient enrollment.
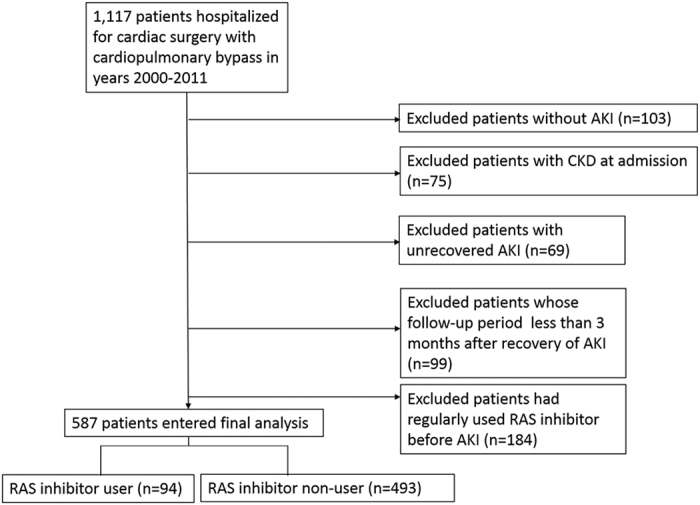
Patients hospitalized between January 1, 2000 and December 31, 2011 were screened using inclusion and exclusion criteria. Totally 587 patients were identified for final analysis. Abbreviation: AKI, acute kidney injury; CKD, chronic kidney disease; RAS, renin-angiotensin system.
The clinical characteristics of the study population were shown in Table 1. Mean age of the patients was 61.8 years old and 69.2% were male. Most patients experienced stage 1 AKI (91.8%). Nearly 40% patients received coronary artery bypass grafting (CABG). Coronary artery disease (CAD, 62.4%) and hypertension (HTN, 54.0%) were the two major comorbidities. HTN was noted in 93.6% and 46.5% of users and non-users of RAS inhibitor, respectively (P < 0.001). At discharge from hospital, 87.2% of users and 26.8% of non-users had treatment with anti-HTN agents (P < 0.001). In contrast, more non-users of RAS inhibitor had congestive heart failure (CHF), metastatic cancer and immunosuppressant treatment.
Table 1. Baseline clinical characteristics of patients.
| Overall (n = 587) | Users of RAS inhibitor (n = 94) | Non-users of RAS inhibitor (n = 493) | P value | |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age, years | 61.8 (14.8) | 60.9 (12.3) | 62.0 (15.2) | 0.45 |
| Man, N (%) | 406 (69.2%) | 69 (73.4%) | 337 (68.4%) | 0.33 |
| Diabetes mellitus, N (%) | 184 (31.4%) | 31 (33.0%) | 153 (31.0%) | 0.71 |
| Hypertension, N (%) | 317 (54.0%) | 88 (93.6%) | 229 (46.5%) | < 0.001 |
| Congestive heart failure NYHA III or IV, N (%) | 191 (32.5%) | 21 (22.3%) | 170 (34.5%) | 0.02 |
| Coronary artery disease, N (%) | 366 (62.4%) | 66 (70.2%) | 300 (60.9%) | 0.09 |
| Peripheral arterial occlusive disease, N (%) | 54 (9.2%) | 10 (10.6%) | 44 (8.9%) | 0.60 |
| Hyperlipidemia, N (%) | 163 (27.8%) | 28 (29.8%) | 135 (27.4%) | 0.63 |
| Chronic obstructive pulmonary disease, N (%) | 70 (11.9%) | 11 (11.7%) | 59 (12.0%) | 0.94 |
| Chronic hepatitis, N (%) | 16 (2.7%) | 1 (1.1%) | 15 (3.0%) | 0.28 |
| Hyperuricemia, N (%) | 81 (13.8%) | 10 (10.6%) | 71 (14.4%) | 0.33 |
| Metastatic cancer, N (%) | 140 (23.9%) | 19 (20.2%) | 121 (24.5%) | 0.37 |
| Current or former smoker, N (%) | 50 (8.5%) | 11 (11.7%) | 39 (7.9%) | 0.23 |
| Laboratory data | ||||
| Baseline hemoglobin, g/dl (SD) | 13.2 (1.8) | 13.5 (1.6) | 13.2 (1.8) | 0.09 |
| Baseline albumin, g/dl (SD) | 4.2 (0.5) | 4.3 (0.5) | 4.2 (0.5) | 0.55 |
| Baseline SCr, mg/dl (SD) | 0.85 (0.18) | 0.87 (0.16) | 0.85 (0.19) | 0.28 |
| Baseline eGFR, ml/min/1.73 m2 (SD) | 89.0 (25.2) | 86.3 (18.4) | 89.5 (26.3) | 0.15 |
| SCr at AKI, mg/dl (SD) | 1.65 (1.01) | 1.61 (0.85) | 1.66 (1.04) | 0.60 |
| SCr at AKI recovery, mg/dl (SD) | 0.91 (0.06) | 0.94 (0.17) | 0.91 (0.08) | 0.14 |
| Urine protein at AKI (severe), N (%) | 28 (4.8%) | 7 (7.5%) | 21 (4.3%) | 0.18 |
| AKI stage, N (%) | ||||
| Stage I | 539 (91.8%) | 89 (94.7%) | 450 (91.3%) | 0.37 |
| Stage II + Stage III | 48 (8.2%) | 5 (5.3%) | 43 (8.7%) | 0.37 |
| Surgical procedure, N (%) | ||||
| Coronary artery bypass grafting | 232 (39.5%) | 38 (40.4%) | 194 (39.4%) | 0.94 |
| Valve surgery | 217 (37.0%) | 28 (29.8%) | 189 (38.3%) | 0.15 |
| Heart transplant | 37 (6.3%) | 2 (2.1%) | 35 (7.1%) | 0.11 |
| Other cardiac surgery | 101 (17.2%) | 26 (27.7%) | 75 (15.2%) | 0.005 |
| Medication at discharge, N (%) | ||||
| Anti-HTN agents | 214 (36.5%) | 82 (87.2%) | 132 (26.8%) | <0.001 |
| Statins | 149 (25.4%) | 28 (29.8%) | 121 (24.5%) | 0.28 |
| Immunosuppressants | 37 (6.3%) | 2 (2.1%) | 35 (7.1%) | 0.07 |
Abbreviation: AKI, acute kidney injury; eGFR, estimated glomerular filtration rate; HTN, hypertension; NYHA, New York Heart Association; RAS, renin-angiotensin system; SCr, serum creatinine.
In the follow-up period after complete renal recovery from AKI, there was no significant difference of SCr at AKI recovery between two groups (Table 1). Of all patients, 39.7% developed CKD which was determined by eGFR <60 ml/min/1.73 m2 (Table 2). In users of RAS inhibitor, 26.6% developed CKD, which was much less than 42.2% in non-users (P = 0.005). The median CKD-free survival time 1079 days in users was much longer than 520 days in non-users (P = 0.011).
Table 2. Development of chronic kidney disease during follow-up.
| Overall (n = 587) | Users of RAS inhibitor (n = 94) | Non-users of RAS inhibitor (n = 493) | P value | |
|---|---|---|---|---|
| CKD development, N (%) | 233 (39.7%) | 25 (26.6%) | 208 (42.2%) | 0.005 |
| Median CKD-free survival time, days | 574 | 1079 | 520 | 0.011 |
Abbreviation: CKD, chronic kidney disease.
Cox Regression Analyses of Risk Factors for CKD Development
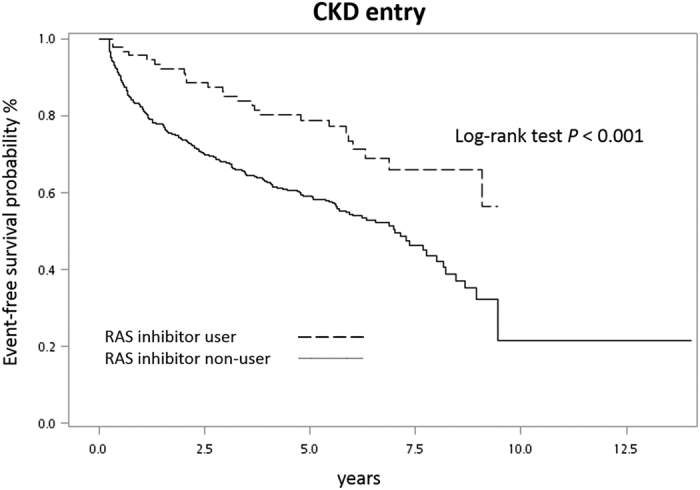
We then performed univariate and multivariate Cox regression analyses to identify independent factors for ensuing CKD development (Table 3). Notably, use of RAS inhibitor was independently associated with lower risk (hazard ratio [HR] = 0.46, P < 0.001). The other factors significantly protective against ensuing CKD were higher levels of baseline hemoglobin and eGFR. Conversely, variables significantly associated with risk of ensuing CKD included older patients, higher SCr level at AKI, diabetes mellitus (DM), CHF, HTN and hyperuricemia. The Kaplan-Meier curve revealed significant protection of RAS inhibitor from ensuing CKD again (Fig. 2).
Table 3. Cox regression analyses for independent factors associated with CKD development.
| Covariate | Univariate Analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Demographic characteristics | ||||
| Age | 1.04 (1.03–1.05) | <0.001 | 1.03 (1.02–1.05) | <0.001 |
| Sexa | 0.81 (0.62–1.06) | 0.12 | ||
| Diabetes mellitusb | 1.99 (1.53–2.57) | <0.001 | 1.61 (1.23–2.10) | 0.001 |
| Hypertensionb | 1.56 (1.20–2.02) | 0.001 | 1.48 (1.12–1.95) | 0.006 |
| Congestive heart failure NYHA III or IVb | 1.38 (1.06–1.81) | 0.02 | 1.38 (1.05–1.81) | 0.02 |
| Coronary artery diseaseb | 1.23 (0.93–1.61) | 0.15 | ||
| Peripheral arterial occlusive diseaseb | 1.44 (0.94–2.21) | 0.09 | ||
| Hyperlipidemiab | 0.99 (0.75–1.31) | 0.95 | ||
| Chronic obstructive pulmonary diseaseb | 1.01 (0.67–1.52) | 0.96 | ||
| Chronic hepatitisb | 1.78 (0.88–3.60) | 0.11 | ||
| Hyperuricemiab | 1.97 (1.44–2.70) | <0.001 | 1.64 (1.19–2.27) | 0.003 |
| Metastatic cancerb | 0.96 (0.71–1.30) | 0.79 | ||
| Current or former smokerb | 0.97 (0.61–1.55) | 0.90 | ||
| Laboratory data | ||||
| Baseline hemoglobin, g/dl | 0.86 (0.80–0.93) | <0.001 | 0.91 (0.84–0.99) | 0.02 |
| Baseline albumin, g/dl | 0.68 (0.52–0.89) | 0.005 | 1.17 (0.86–1.59) | 0.32 |
| Baseline SCr, mg/dl | 8.72 (4.09–18.58) | <0.001 | ||
| Baseline eGFR, ml/min/1.73 m2 | 0.96 (0.96–0.97) | <0.001 | 0.98 (0.97–0.99) | <0.001 |
| SCr at AKI, mg/dl | 1.47 (1.35–1.60) | <0.001 | 1.52 (1.36–1.70) | <0.001 |
| Urine protein at AKIc | 2.25 (1.37–3.69) | 0.001 | 1.37 (0.81–2.32) | 0.24 |
| AKI staged | 2.33 (1.57–3.44) | <0.001 | ||
| Surgical procedure | ||||
| Coronary artery bypass graftinge | 1.35 (0.92–1.97) | 0.13 | ||
| Valve surgerye | 0.85 (0.57–1.28) | 0.43 | ||
| Heart transplante | 1.47 (0.84–2.58) | 0.18 | ||
| Medication | ||||
| RAS inhibitorb | 0.47 (0.31–0.72) | <0.001 | 0.46 (0.30–0.70) | <0.001 |
| Anti-HTN agentsb | 0.99 (0.76–1.29) | 0.92 | ||
| Statinsb | 0.94 (0.70–1.25) | 0.66 | ||
| Immunosuppressantsb | 1.19 (0.74–1.93) | 0.48 | ||
Annotation: aMan compared to woman; bCompared to no status; cSevere compared to mild; dStage II + III compared to stage I; eCompared to other cardiac surgery.
Abbreviation: CI, confidence interval; HR, hazard ratio.
Figure 2. Kaplan-Meier analysis of CKD-free-survival for users and non-users of RAS inhibitor.
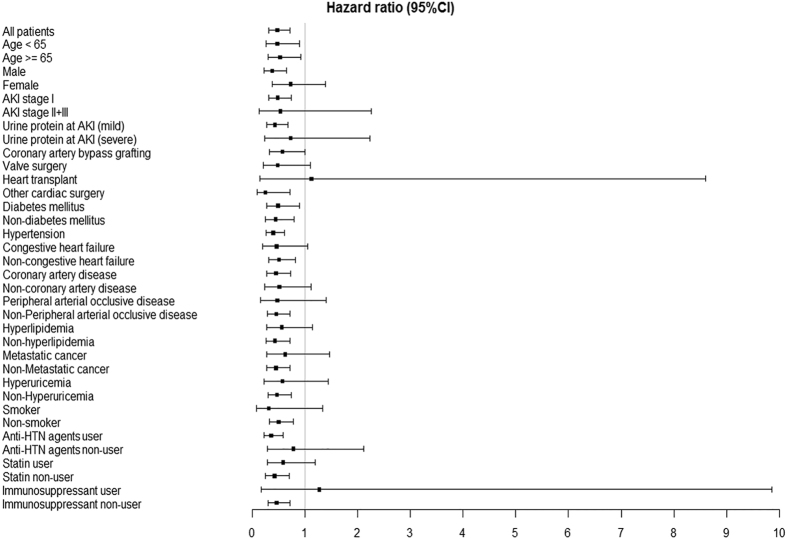
We further performed subgroup analyses (Fig. 3). Use of RAS inhibitor was still significantly associated with lower risk of ensuing CKD in most subgroups except female patients, patients with AKI stage II/III, severe proteinuria at AKI, peripheral arterial occlusive disease (PAOD), hyperlipidemia, hyperuricemia, metastatic cancer, non-users of anti-HTN agents, users of statins or immunosuppressants, smokers and heart transplant recipients. RAS inhibitor had borderline protective effect on subgroups of patients undergoing CABG or valve surgery as well as patients with CHF and non-coronary artery disease. On the other hand, the HR of ensuing CKD was much lower in several subgroups, including male patients, other cardiac surgery and anti-HTN agent users.
Figure 3. Hazard ratio (95% confidence interval) for ensuing CKD associated with use of RAS inhibitor in subgroups of enrolled patients.
Abbreviation: CI, confidence interval; HTN, hypertension.
Discussion
There is substantial progress in the field of AKI over the past 10 years23,24. The previous conventional wisdom that AKI survivors with complete renal recovery tend to enjoy good health appears to be flawed6,25,26,27,28. Most of the previous studies had various definition of renal recovery, including weaning from dialysis, return of eGFR to >90% of reference or return of SCr to within 20% of baseline, etc.29,30. Although the Acute Dialysis Quality Initiative (ADQI) consensus defines complete renal recovery as return to baseline classification within the RIFLE criteria and partial recovery as a change in RIFLE status in patient free of dialysis31, few studies have evaluated renal recovery in accordance with this recommendation. Even KDOQI guideline has no definition of complete renal recovery. The lack of a consistent definition for renal recovery is an obstacle for adequate comparison between studies for incidence of subsequent CKD and to develop strategies for patient monitoring and treatment after AKI. Therefore, renal recovery defined as the decrease of SCr level to within 0.3 mg/dl above the baseline in our patients without CKD history before AKI is probably most close to concept of complete renal recovery in ADQI consensus. Moreover, it is important to clarify the outcome of these patients with complete renal recovery, who are thought to do well and lack of continuous monitoring of renal function in general. After AKI, we can treat patients without complete renal recovery as CKD, but we may miss the golden time to prevent the development and progression of ensuing CKD in patients with complete renal recovery if we pursue the conventional wisdom.
Our results confirmed that 39.7% of CSA-AKI survivors with complete renal recovery developed CKD during median follow-up duration of 2.99 years. Use of RAS inhibitor after complete renal recovery from CSA-AKI was strikingly associated with risk reduction by 54% for ensuing CKD development after multivariate adjustment. To our knowledge, this is the first clinical report to confirm the association of RAS inhibitor with the risk reduction for ensuing CKD in patients with complete renal recovery from AKI. Moreover, this clinical study confirms our previous study in mice that RAS inhibition by losartan can reduce ensuing CKD and mortality after functional recovery from AKI induced by ischemia-reperfusion injury20. To minimize the impact of different etiologies of AKI on ensuing CKD development and on the protective effect of RAS inhibitor, we chose patients undergoing cardiac surgery with cardiopulmonary bypass (CPB) as the study population from our prospectively collected observational cohort6,13,28,32,33,34,35,36. CSA-AKI is the second most common cause of AKI in the intensive care unit37. Ischemia-reperfusion injury, similar to the mechanism responsible for the animal model used in our recent study regarding RAS inhibition on AKI-CKD transition20, is thought to play a major role in the pathogenesis of CSA-AKI38,39. The striking and consistent effect on risk reduction for ensuing CKD development in clinical cohort and mouse model of AKI-CKD continuum provides evidence for clinical application of RAS inhibitor to prevent ensuing CKD development in AKI survivors with complete renal recovery even patients do not have CKD history before AKI.
Burgeoning studies have shown that mild AKI can increase the risk of CKD and mortality and postulated a persistent pathophysiological change in kidney even patients achieve functional recovery6,40. Several mechanisms responsible for the AKI-CKD transition have been demonstrated. These mechanisms include tubular cell loss41, tubular cell G2/M cell-cycle arrest15, persistent inflammation42, microvascular rarefaction18,19, and epigenetic change related cell proliferation of pericytes/fibroblasts after AKI17,43,44. Moreover, abnormal renal pathology and ongoing injury are still noted in a murine model even biochemical parameters of renal function have returned to baseline after AKI20. Our previous study has shown the upregulation of genes Agt and Agtr1a that encoded angiotensinogen and angiotensin II type 1a receptor respectively in injured kidneys, suggesting ongoing activation of intrarenal RAS20. It is noteworthy that some studies indicate the activation of RAS after AKI. In CSA-AKI, low cardiac output before, during, or after surgery is directly related to AKI risk due to increased renal vasoconstriction via RAS activation45. In addition, overexpression of intrarenal RAS is reported in patients with acute tubular necrosis and is associated with the severity of AKI and urinary levels of angiotensinogen reflect intrarenal RAS activity46,47,48. Incomplete tubular epithelial regeneration results in nephron loss and hyperfiltration in the remaining glomeruli49. RAS activation is the plausible cause for this change to maintain glomerular filtration after AKI. This mechanism appears to be one of the mechanisms for the elevated blood pressure after AKI in a recent clinical study as well50. Many clinical trials have proved the specific renoprotective effect of RAS inhibition by ACE inhibitor/ARB for patients with diabetic or proteinuric non-diabetic CKD to reduce disease progression and mortality51,52,53. However, RAS inhibition is usually avoided during the acute phase of AKI patients, and the role of RAS activity in acute phase and injury severity is not clear indeed21,22,54. Based on findings that intrarenal RAS was activated in repairing kidneys in spite of complete recovery of plasma parameters for renal function assessment one month after acute injury, our previous study has shown that RAS inhibition with losartan in mouse AKI survivors can prevent the development of ensuing CKD and mortality20. Furthermore, focal tubular atrophy, ongoing inflammation, and intrarenal RAS activation led to a vicious cycle in repairing kidneys for ensuing CKD progression even plasma biochemical parameters showed recovery from AKI. Evidence becomes more clear that RAS inhibitor can provide a key to break the vicious cycle for AKI-CKD transition.
Moreover, RAS inhibitors may prevent ensuing CKD indirectly thorough reduction of cardiorenal syndrome55. RAS inhibitor therapy has been included in major society guideline of heart failure management56, because multiple clinical trials have shown that RAS inhibitor therapy leads to symptomatic improvement, reduced hospitalization, and lower mortality in patients with heart failure57. This cardiovascular protective effect could reduce acute or chronic cardiorenal syndrome related ensuing CKD.
Our analyses confirmed that traditional risk factors for CKD development58,59, such as old age, DM, higher SCr at AKI, HTN, CHF and hyperuricemia have significant impact for AKI-CKD transition. Our analyses also showed that higher hemoglobin and baseline eGFR can protect from ensuing CKD, possibly through reducing hypoxia during AKI and higher renal reserve as reported previously60,61.
Our subgroup analyses showed that groups of male patients, other cardiac surgery and anti-HTN agents acquire a higher magnitude of benefits from RAS inhibitor than all patients, suggesting higher RAS activity in these subgroups. On the contrary, some subgroups had no significant risk reduction of CKD development even under RAS inhibition. In addition to RAS activation, another dominant mechanism need to be unraveled for AKI-CKD transition in groups of female patients, severe AKI (stage II/III), severe proteinuria at AKI, PAOD, hyperuricemia, hyperlipidemia, smokers, metastatic cancer and non-users of anti-HTN agents. No benefits for heart transplant recipients and statins or immunosuppressant users could be due to different pathogenesis of its correspondingly underlying disease or its specific drug effect.
There are some limitations in our study. First, this was an observational study. Well designed-clinical trials should be initiated in the future to prove the protective effect of RAS inhibitor on ensuing CKD after complete renal recovery from AKI. Second, 10.2% of patients without CKD development received follow-up period less than one year which may underestimate the incidence of ensuing CKD after AKI. It might take longer time for our patients to develop CKD because most patients had mild AKI and median duration required for ensuing CKD development was far more than one year. Third, only patients with CSA-AKI were included. To extend our findings, we need more studies to investigate the effect of RAS inhibitor after complete renal recovery from AKI due to the other mechanisms. Finally, urinalysis was not checked as frequently as SCr. Therefore, any abnormal findings in urine representing the residual renal damage in patients could not be timely noticed. Even though these limitations, this prospectively collected observational cohort study provided the strong evidence that use of RAS inhibitor after complete renal recovery from CSA-AKI was associated with risk reduction by 54% for ensuing CKD after multivariate adjustment.
In conclusion, in patients without CKD history, use of ACE inhibitor or ARB was associated with lower risk of ensuing CKD development after complete renal recovery from CSA-AKI. Our study highlights the important role of RAS activation in AKI-CKD transition. Use of RAS inhibitor should be included in the strategies for post-AKI care.
Methods
Patients
This was a prospectively collected observational cohort study based on the National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF) database established in the surgical intensive care units (SICU). We screened patients in this database who were ≥18 years old and hospitalized in the SICU of National Taiwan University Hospital (NTUH) for postoperative care of cardiac surgery with CPB between January 1, 2000 and December 31, 2011. We excluded patients who did not have AKI after surgery. Other exclusion criteria were history of CKD, preoperative eGFR <60 ml/min/1.73 m2 by Taiwanese MDRD equation62, no recovery of SCr to level within 0.3 mg/dl above baseline within one month after AKI, follow-up period less than 3 months after AKI, and medication with RAS inhibitor including ACE inhibitor or ARB before AKI. The follow-up was continued until September 30, 2016.
The study was approved by the Institutional Review Board of NTUH. A waiver of informed consent was obtained because there was no breach of privacy and no interference with patient care.
Clinical Assessment of Patients
Preoperative demographic data were obtained at SICU admission. These variables included age, gender, smoking history, DM (using oral hypoglycemic agents or insulin), HTN (using anti-HTN agents or systolic/diastolic blood pressure >140/90 mmHg at admission), CHF (defined as New York Heart Association (NYHA) functional class III or IV), PAOD (defined by clinical or imaging diagnosis), chronic obstructive pulmonary disease (with long-term bronchodilators), hyperlipidemia (with lipid-lowering agents) and CAD (defined by the diagnostic code of ischemic heart disease prior to admission, and positive electrocardiographic findings). Chronic hepatitis and metastatic cancer were also recorded according to diagnostic codes prior to admission.
Laboratory data such as baseline SCr, albumin, hemoglobin were recorded at SICU admission. SCr at the peak of AKI was also obtained. Urine protein at AKI was recorded according to urine dipstick test. Mild proteinuria and severe proteinuria were defined by the result of trace to 1 + and 2 + to 4 + respectively. Baseline eGFR was calculated using Taiwanese MDRD equation62. The surgical procedure was categorized into CABG, heart valve surgery, heart transplant and others. The AKI definition and staging were based on Kidney Disease: Improving Global Outcomes (KDIGO) criteria63.
Medications such as anti-HTN agents (including calcium channel blockers, β-blockers, α-blockers, clonidine), lipid-lowering agents (statins), immunosuppressants after heart transplant were recorded during hospitalization and at discharge. Users of RAS inhibitor were defined as starting ACE inhibitor or ARB between 1 and 6 months after complete renal recovery from AKI and continuing the medication during follow-up, while the others were defined as non-users.
In addition to data collection during hospitalization and at discharge, we also leveraged an electric medical record to keep track of important covariates when patient visited our outpatient department, including SCr, medications and diagnosis of comorbidity.
Outcome
The endpoint was stage 3 CKD development during the follow-up period. All of the longitudinal measurements of SCr and eGFR during hospitalization and follow-up period were obtained for each enrolled patient. Stage 3 CKD was determined by eGFR below 60 ml/min/1.73 m2 62. Patient who lost follow-up before September 30, 2016 would be seen as censored data.
Statistical Analysis
We performed statistical analyses with the SAS software, version 9.4. Continuous variables were presented as mean (standard deviation, SD), and the difference between users and non-users of RAS inhibitor was compared with the Student’s t-test. Categorical variables were summarized as percentages and analyzed with the chi-square test. Two-sided P < 0.05 was considered statistically significant. We constructed a univariate and multivariate Cox regression model to investigate the association between use of RAS inhibitor and CKD development. Variables significantly associated with CKD development in the univariate analysis (P < 0.05) were included in the multivariate Cox regression model. The survival curves for CKD development were plotted using Kaplan-Meier method. Subgroup analyses were also performed to estimate the HR. Subgroups of variables deemed clinical relevant to CKD development, which included age, sex, DM, HTN, CHF, CAD, PAOD, hyperlipidemia, hyperuricemia, metastatic cancer, smoking status, AKI stage, urine protein at AKI, surgical procedures, medication use such as anti-HTN agents, statins or immunosuppressants were identified.
Additional Information
How to cite this article: Chou, Y.-H. et al. Renin-Angiotensin System Inhibitor is Associated with Lower Risk of Ensuing Chronic Kidney Disease after Functional Recovery from Acute Kidney Injury. Sci. Rep. 7, 46518; doi: 10.1038/srep46518 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
Y.H.C. is supported by Ministry of Science and Technology, Taiwan (MOST, 104-2314-B-002-119) and Mrs. Hsiu-Chin Lee Kidney Research Foundation. S.L.L. is supported by MOST (105-2314-B-002-096-MY3), National Health Research Institutes (NHRI-EX106-10633SI) and NTUH (106-P02, 105-S2944). We thank all NSARF members for their contribution in the setup and maintenance of database.
Footnotes
The authors declare no competing financial interests.
Author Contributions Y.H.C., T.M.H., S.Y.P., C.F.L. and V.C.W. carried out data collection and analyzed data. C.H.C. participated in data analysis. M.S.W., K.D.W., T.S.C. and S.L.L. designed and directed the project, carried out experiments, analyzed data and wrote the manuscript. All authors reviewed and approved the manuscript as submitted.
References
- Lafrance J. P. & Miller D. R. Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 21, 345–352 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L. et al. Acute kidney injury in China: a cross-sectional survey. Lancet 386, 1465–1471 (2015). [DOI] [PubMed] [Google Scholar]
- Xu X. et al. Epidemiology and clinical correlates of AKI in Chinese hospitalized adults. Clin J Am Soc Nephrol 10, 1510–1518 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta R. L. et al. Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 Global Snapshot: a multinational cross-sectional study. Lancet 387, 2017–2025 (2016). [DOI] [PubMed] [Google Scholar]
- Chawla L. S. & Kimmel P. L. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 82, 516–524 (2012). [DOI] [PubMed] [Google Scholar]
- Lai C. F. et al. Kidney function decline after a non-dialysis-requiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care 16, R123 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla L. S., Eggers P. W., Star R. A. & Kimmel P. L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371, 58–66 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer E. et al. Urinary neutrophil gelatinase-associated lipocalin distinguishes pre-renal from intrinsic renal failure and predicts outcomes. Kidney Int 80, 405–414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakar C. V., Christianson A., Himmelfarb J. & Leonard A. C. Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephol 6, 2567–2572 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heung M. et al. Acute kidney injury recovery pattern and subsequent risk of CKD: An analysis of Veterans Health Administration Data. Am J Kidney Dis 67, 742–752 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y. H., Pan S. Y., Yang C. H. & Lin S. L. Stem cells and kidney regeneration. J Formos Med Assoc 113, 201–209 (2014). [DOI] [PubMed] [Google Scholar]
- Chou Y. H. et al. The role of brain natriuretic peptide in predicting renal outcome and fluid management in critically ill patients. J Formos Med Assoc 114, 1187–1196 (2015). [DOI] [PubMed] [Google Scholar]
- Chou Y. H. et al. Impact of timing of renal replacement therapy initiation on outcome of septic acute kidney injury. Crit Care 15, R134 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferenbach D. A. & Bonventre J. V. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol 11, 264–276 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Besschetnova T. Y., Brooks C. R., Shah J. V. & Bonventre J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med 16, 535–543 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. T. et al. Platelet-derived growth factor receptor signaling activates pericyte-myofibroblast transition in obstructive and post-ischemic kidney fibrosis. Kidney Int 80, 1170–1181 (2011). [DOI] [PubMed] [Google Scholar]
- Wu C. F. et al. Transforming growth factor beta-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol 182, 118–131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbelt M. et al. Acute and chronic microvascular alterations in a mouse model of ischemic acute kidney injury. Am J Physiiol Renal Physiol 293, F688–695 (2007). [DOI] [PubMed] [Google Scholar]
- Lin S. L. et al. Targeting endothelium-pericyte cross talk by inhibiting VEGF receptor signaling attenuates kidney microvascular rarefaction and fibrosis. Am J Pathol 178, 911–923 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S. Y. et al. Losartan reduces ensuing chronic kidney disease and mortality after acute kidney injury. Sci Rep 6, 34265 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romo R. et al. AT1 receptor antagonism before ischemia prevents the transition of acute kidney injury to chronic kidney disease. Kidney Int 89, 363–373 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang J. et al. Competing actions of type 1 angiotensin II receptors expressed on T lymphocytes and kidney epithelium during cisplatin-induced AKI. J Am Soc Nephrol 27, 2257–2264 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuk A. & Bonventre J. V. Acute kidney injury. Annu Rev Med 67, 293–307 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rewa O. & Bagshaw S. M. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 10, 193–207 (2014). [DOI] [PubMed] [Google Scholar]
- Finkenstaedt J. T. & Merrill J. P. Renal function after recovery from acute renal failure. N Engl J Med 254, 1023–1026 (1956). [DOI] [PubMed] [Google Scholar]
- Newsome B. B., Warnock D. G. & McClellan W. M. et al. Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med 168, 609–616 (2008). [DOI] [PubMed] [Google Scholar]
- Ishani A. et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 171, 226–233 (2011). [DOI] [PubMed] [Google Scholar]
- Wu V. C. et al. Long-term risk of coronary events after AKI. J Am Soc Nephrol 25, 595–605 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo E., Zanetta D. M. & Abdulkader R. C. Long-term follow-up of patients after acute kidney injury: patterns of renal functional recovery. PloS one 7, e36388 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo E., Bouchard J. & Mehta R. L. Renal recovery following acute kidney injury. Curr Opin Crit Care 14, 660–665 (2008). [DOI] [PubMed] [Google Scholar]
- Bellomo R. Defining, quantifying, and classifying acute renal failure. Crit Care Clin 21, 223–237 (2005). [DOI] [PubMed] [Google Scholar]
- Huang T. M. et al. Preoperative proteinuria predicts adverse renal outcomes after coronary artery bypass grafting. J Am Soc Nephrol 22, 156–163 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao C. C. et al. Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care 13, R171 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiao C. C. et al. U-curve association between timing of renal replacement therapy initiation and in-hospital mortality in postoperative acute kidney injury. PloS one 7, e42952 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu V. C. et al. The impact of acute kidney injury on the long-term risk of stroke. J Am Heart Assoc 3, e000933 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu V. C. et al. Acute-on-chronic kidney injury at hospital discharge is associated with long-term dialysis and mortality. Kidney Int 80, 1222–1230 (2011). [DOI] [PubMed] [Google Scholar]
- Uchino S. et al. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294, 813–818 (2005). [DOI] [PubMed] [Google Scholar]
- Thiele R. H., Isbell J. M. & Rosner M. H. AKI associated with cardiac surgery. Clin J Am Soc Nephrol 10, 500–514 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner M. H., Portilla D. & Okusa M. D. Cardiac surgery as a cause of acute kidney injury: pathogenesis and potential therapies. J Intensive Care Med 23, 3–18 (2008). [DOI] [PubMed] [Google Scholar]
- Chertow G. M., Burdick E., Honour M., Bonventre J. V. & Bates D. W. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16, 3365–3370 (2005). [DOI] [PubMed] [Google Scholar]
- Endo T. et al. Exploring the origin and limitations of kidney regeneration. J Pathol 236, 251–263 (2015). [DOI] [PubMed] [Google Scholar]
- Ko G. J. et al. Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol 298, F1472–1483 (2010). [DOI] [PubMed] [Google Scholar]
- Bechtel W. et al. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat Med 16, 544–550 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. T. et al. DNA methyltransferase inhibition restores erythropoietin production in fibrotic murine kidneys. J Clin Invest 126, 721–731 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo R. et al. The pathophysiology of cardiac surgery-associated acute kidney injury (CSA-AKI). Int J Artif Organs 31, 166–178 (2008). [DOI] [PubMed] [Google Scholar]
- Cao W. et al. Overexpression of intrarenal renin-angiotensin system in human acute tubular necrosis. Kidney Blood Press Res 41, 746–756 (2016). [DOI] [PubMed] [Google Scholar]
- Rosen S. & Stillman I. E. Acute tubular necrosis is a syndrome of physiologic and pathologic dissociation. J Am Soc Nephrol 19, 871–875 (2008). [DOI] [PubMed] [Google Scholar]
- Yang X. et al. Urinary angiotensinogen level predicts AKI in acute decompensated heart failure: a prospective, two-stage study. J Am Soc Nephrol 26, 2032–2041 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A. et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298, F1078–1094 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu C. Y. et al. Elevated BP after AKI. J Am Soc Nephrol 27, 914–923 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar M. Z. et al. Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker use, and mortality in patients with chronic kidney disease. J Am Coll Cardiol 63, 650–658 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 345, 851–860 (2001). [DOI] [PubMed] [Google Scholar]
- Brenner B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345, 861–869 (2001). [DOI] [PubMed] [Google Scholar]
- Huang T. M. et al. Association of pre-operative ACEIs or ARBs with a reduction in post-operative AKI after elective CABG. J Am Soc Nephrol 27, 75A (2010). [Google Scholar]
- Ronco C., Haapio M., House A. A., Anavekar N. & Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 52, 1527–1539 (2008). [DOI] [PubMed] [Google Scholar]
- Yancy C. W. et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 62, e147–239 (2013). [DOI] [PubMed] [Google Scholar]
- Lund L. H., Benson L., Dahlstrom U. & Edner M. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction. JAMA 308, 2108–2117 (2012). [DOI] [PubMed] [Google Scholar]
- McClellan W. M. & Flanders W. D. Risk factors for progressive chronic kidney disease. J Am Soc Nephrol 14, S65–70 (2003). [DOI] [PubMed] [Google Scholar]
- Li L. et al. Is hyperuricemia an independent risk factor for new-onset chronic kidney disease?: A systematic review and meta-analysis based on observational cohort studies. BMC Nephrol 15, 122 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh M. et al. The association between perioperative hemoglobin and acute kidney injury in patients having noncardiac surgery. Anesth Analg 117, 924–931 (2013). [DOI] [PubMed] [Google Scholar]
- Sawhney S., Mitchell M., Marks A., Fluck N. & Black C. Long-term prognosis after acute kidney injury (AKI): what is the role of baseline kidney function and recovery? A systematic review. BMJ Open 5, e006497 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L. I. et al. Modification of diet in renal disease (MDRD) study and CKD epidemiology collaboration (CKD-EPI) equations for Taiwanese adults. PloS one 9, e99645 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusa M. D. & Davenport A. Reading between the (guide)lines–the KDIGO practice guideline on acute kidney injury in the individual patient. Kidney Int 85, 39–48 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]