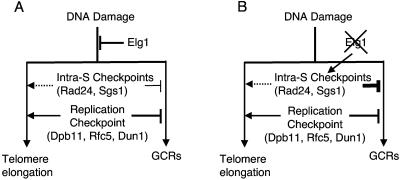
FIG. 5.
Hypothetical model how Elg1, the intra-S checkpoints, and the DNA replication checkpoint function together to suppress GCRs and telomere elongation. (A) Elg1, the intra-S checkpoint, and the DNA replication checkpoint all function to suppress GCRs at different levels redundantly. Unlike Elg1, which functions to suppress telomere elongation, the DNA replication checkpoint is required for the telomere elongation in the absence of Elg1. The intra-S checkpoint seems also to be involved in telomere elongation (dashed line). (B) In the absence of Elg1, the intra-S checkpoint is highly activated because of DNA damage. This is why the large increase in GCR formation observed in strains defective in the DNA replication checkpoint is suppressed by the elg1Δ mutation. Telomere size is increased because of the lack of Elg1, but in the absence of the DNA replication checkpoint, telomere size was not increased or was only slightly increased by the elg1Δ mutation since the DNA replication checkpoint is required for telomere elongation by the elg1Δ mutation (solid line arrow). An additional mutation in the intra-S checkpoint in strains carrying elg1Δ and a mutation in genes for the DNA replication checkpoint (dpb11-1 and dun1Δ) further decreased telomere size. This suggests that the intra-S checkpoint also seems to function in telomere elongation in the absence of Elg1 (dashed line arrow).