Figure 1. Cytosolic formation of fluorescent puncta composed of PB1 domain fused to AG.
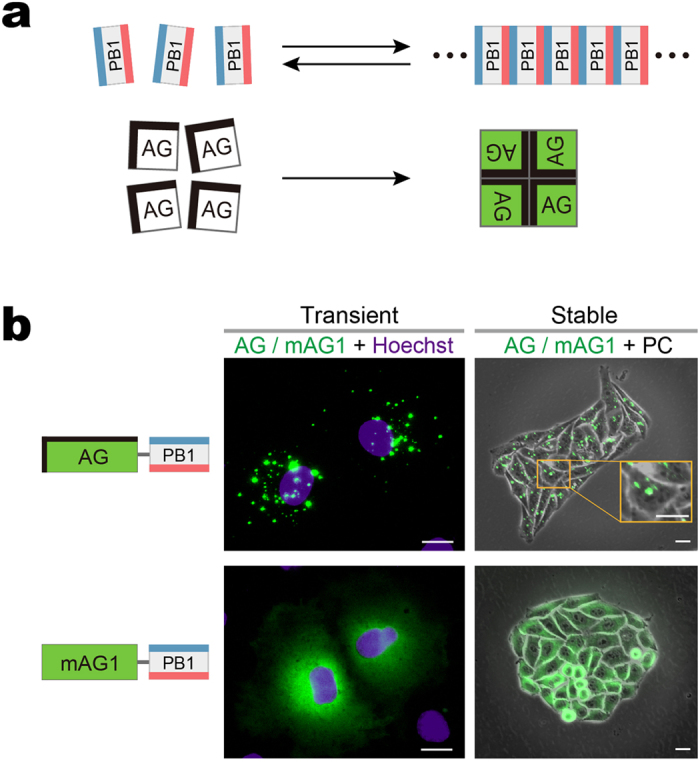
(a) Homo-oligomerization of the p62 PB1 domain (top) and the green-emitting fluorescent protein AG (Azami-Green) (bottom). top, PB1 self-associates in an equilibrium in a front-to-back topology to form a high-molecular-weight homo-oligomer. The conserved acidic/hydrophobic and lysine/arginine residues of PB1 are indicated by red and blue bars, respectively. bottom, AG forms an obligate tetramer complex to become fluorescent (green). The hydrophobic interfaces between AG subunits are indicated by thick bars on two adjacent sides. (b) Fluorescence images of cultured cells expressing PB1 fusion to AG and the monomeric mutant of AG (mAG1), AG-PB1 and mAG1-PB1, respectively. The absence of a thick black bar indicates that mAG1 has no hydrophobic patches on its surface. (AG/mAG1 + Hoechst) Fluorescence images of Cos-7 cells transiently expressing AG-PB1 (upper) and mAG1-PB1 (lower) one day post-transfection. Images of AG/mAG1 (green) and Hoechst33342-stained nuclei (violet) were merged. The same distribution pattern was observed in approximately 50 other cells for each construct. (AG/mAG1 + PC) Fluorescence images of HeLa cells stably expressing AG-PB1 (upper) and mAG1-PB1 (lower). Their phase-contrast (PC) images were merged. A high-magnification image is shown to zoom in on fluorescent clusters of AG-PB1 (orange inset). The same distribution pattern was observed in 4 other HeLa cell clones for each construct. Scale bars, 5 μm. See also Supplementary Fig. 1.
